- Smoke Signals: Navigating the Evolving Landscape of Nicotine and Tobacco Regulations
- Posts
- 2024 Nicotine and Tobacco Regulatory and Science Recap
2024 Nicotine and Tobacco Regulatory and Science Recap
Cheers to 2025

2024 was a transformative year professionally and I want to thank all who have subscribed to this newsletter and collaborated on my work. The “why” of the work has remained the same. The fundamental goals continue to center around harm reduction and providing regulatory solutions to products alternative to traditional tobacco products.
The “how” of the work has certainly changed. Accelerating these transformations will continue in 2025. Predictive and Generative AI's role in knowledge synthesis - monitoring and analyzing complex scientific and regulatory developments - will be incredibly impactful to those who embrace it.

U.S. Nicotine and Tobacco Industry Analysis 2024
FDA's PMTA Review: The FDA demonstrated some progress in its review of premarket tobacco product applications (PMTAs), for ENDS products taking action on a substantial portion of e-cigarette product applications. Almost no action was taken on modern oral nicotine pouch PMTAs. Expect that to change in 2025.
Enforcement Focus: The FDA and Department of Justice (DOJ) intensified efforts to combat illicit e-cigarettes, particularly youth-appealing products, through a new task force, warning letters, seizures, and civil monetary penalties.
State-Level Regulations: States maintained a crucial role in tobacco control, with all 50 states implementing youth access restrictions and some exploring e-cigarette registries.
Court Decisions: Federal court decisions, including a Supreme Court case on flavored vape denials, will shape the regulatory landscape and enforcement approaches.
Product Development: The industry pursued product innovations, including rechargeable pods, nicotine-free vapes, and devices with advanced temperature control.
Marketing Shifts: Companies adapted marketing strategies to address regulatory changes and consumer preferences, with an emphasis on online channels and harm reduction messaging.
Regulatory Environment
FDA Actions on PMTAs
In 2024, the FDA made significant progress in its review of PMTAs for e-cigarette products that were on the market as of August 8, 2016. By July 22, 2024, the agency had taken action on 185 out of 186 marketing applications for e-cigarette products with significant market share - the “covered applications” . These actions included issuing marketing denial orders (MDOs) or granting marketing authorizations. Notably, the FDA issued MDOs to Imperial Brands' Fontem US for four blu disposable and one myblu e-cigarette products due to their characterizing flavors, including menthol, mint, vanilla, and cherry.
The FDA rescinded its MDO for all Juul e-cigarette products on June 6, 2024. This action reverted Juul's premarket application to pending status, allowing the company to continue marketing its products while the FDA reevaluates its application. The FDA's decision to rescind the MDO, while seemingly a setback for tobacco control efforts, reflects the complexities of regulating e-cigarettes and the ongoing legal challenges faced by the agency.
In June 2024, the FDA authorized the marketing of four menthol-flavored e-cigarette products from NJOY LLC: NJOY DAILY Menthol and NJOY ACE Menthol. This authorization, granted after an extensive scientific review, marked a significant step in the FDA's regulation of flavored e-cigarettes. The agency emphasized that the authorization included restrictions to mitigate youth risk, reflecting its commitment to balancing harm reduction for adult smokers with youth protection.
Furthermore, the FDA granted marketing orders for Vuse Alto devices and their Golden Tobacco and Rich Tobacco flavor pods at varying nicotine levels (1.8%, 2.4%, and 5%). This authorization further expanded the range of legally marketed e-cigarette products in the U.S.
In a move aimed at enhancing transparency, the FDA released regulatory science policy memos in November 2024, outlining its process for prioritizing and reviewing PMTAs, particularly for flavored e-cigarettes. The memos also provided information on the criteria used for environmental assessments related to e-cigarette products. In addition to the November releases, the FDA also released memos in April, May, and August of 2024. The August releases included a memo on genotoxicity hazard identification and carcinogenicity tiering of constituents in ENDS PMTAs, as well as a memo on calculating excess lifetime cancer risk (ELCR) in ENDS PMTAs.
Enforcement Activities
Warning Letters to Retailers: The FDA issued over 800 warning letters to brick-and-mortar and online retailers for selling unauthorized e-cigarette products.
Seizures: In April 2024, the FDA and DOJ, in coordination with the U.S. Marshals Service, seized over 45,000 unauthorized e-cigarettes valued at more than $700,000 from a warehouse in California.
Civil Monetary Penalties: The FDA sought civil monetary penalties against 140 retailers for continuing to sell unauthorized products, with the total amount exceeding $2,600,000.
Federal Leadership Changes
President-elect Trump's pledge to "save vaping again" suggests potential regulatory easing
Anticipated impacts:
Possible streamlined PMTA process
Potential relaxation of flavor restrictions
Continued focus on youth prevention but with less aggressive enforcement
FDA leadership transition expected to maintain science-based regulation while potentially adopting more industry-friendly stance
ENDS Product Innovation 2024 Developments
Rechargeable pod systems with enhanced capacity and battery life
Advanced temperature control technology
Sustainable/recyclable designs addressing environmental concerns
Expanded nicotine strength options, including nicotine-free alternatives
Smart technology integration for usage monitoring
Focus on regulatory compliance features like age-gating technology

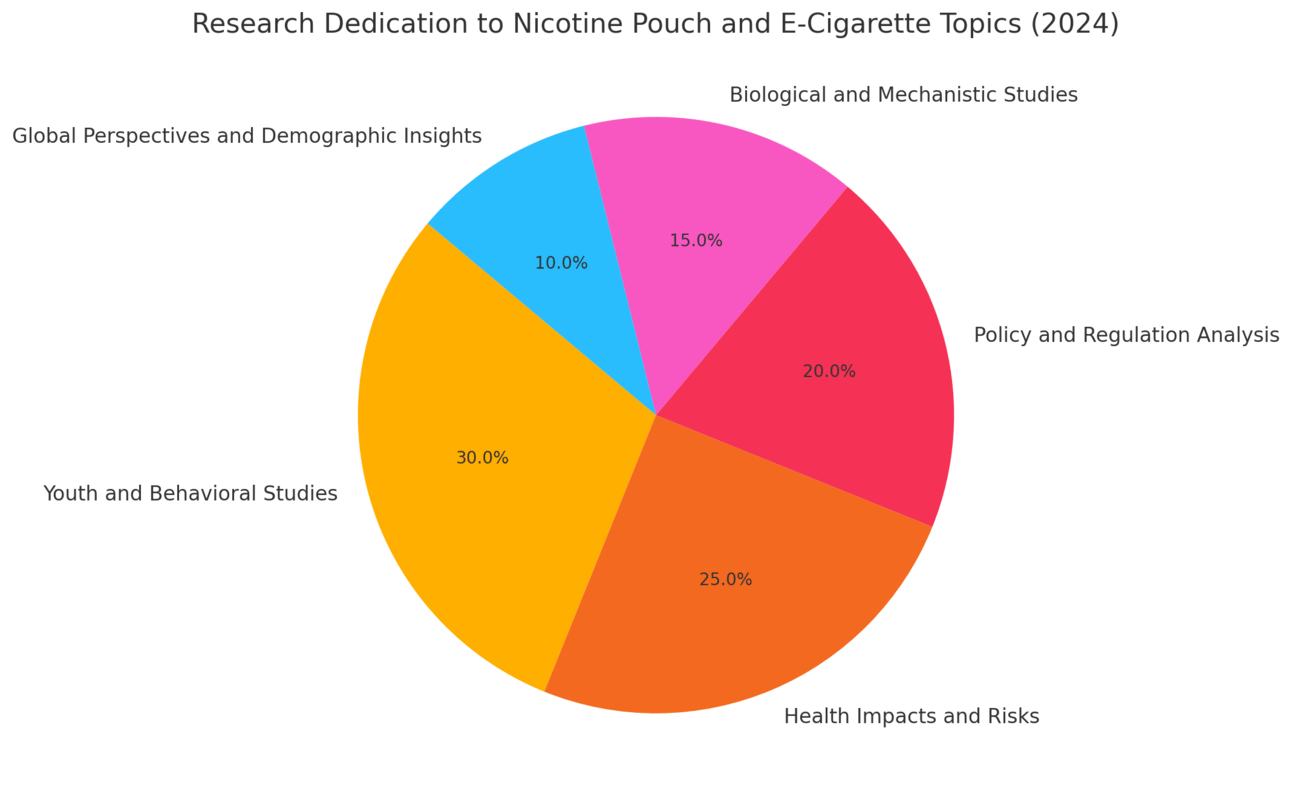
Trends in Nicotine and Tobacco Research for 2024
Key Research Trends:
Youth and Behavioral Studies (30%)
Significant focus on youth use of e-cigarettes and behavioral patterns, including:
Factors influencing underage use of disposable electronic nicotine delivery systems (ENDS).
Influence of social media, marketing, and peer networks on e-cigarette adoption.
Associations between psychosocial factors (e.g., depressive symptoms) and nicotine use in adolescents and young adults.
Health Impacts and Risks (25%)
Studies highlight the adverse effects of nicotine and e-cigarette use, such as:
Pulmonary health impacts (e.g., lung function and oxidative stress).
Cardiovascular risks, particularly LDL-cholesterol elevation in users of heated tobacco products.
Oral health deterioration linked to e-cigarette aerosol exposure.
Policy and Regulation Analysis (20%)
Evaluations of regulatory interventions include:
Impacts of flavor bans and minimum floor price laws on tobacco consumption.
Analysis of stakeholder responses and enforcement challenges in regions implementing regulatory policies.
Biological and Mechanistic Studies (15%)
Exploration of nicotine's biological effects, such as:
Nicotine-induced endothelial dysfunction and its role in exacerbating atherosclerosis.
Impacts on lipid metabolism, pulmonary surfactants, and cellular proliferation pathways.
Global Perspectives and Demographic Insights (10%)
Cross-cultural comparisons and demographic-focused studies examine:
Regional differences in e-cigarette use perception (e.g., US vs. Vietnam).
Increased tobacco use among women in India and its cultural drivers.