- Smoke Signals: Navigating the Evolving Landscape of Nicotine and Tobacco Regulations
- Posts
- 4️⃣ Signals - April 29, 2024
4️⃣ Signals - April 29, 2024
Issue #8
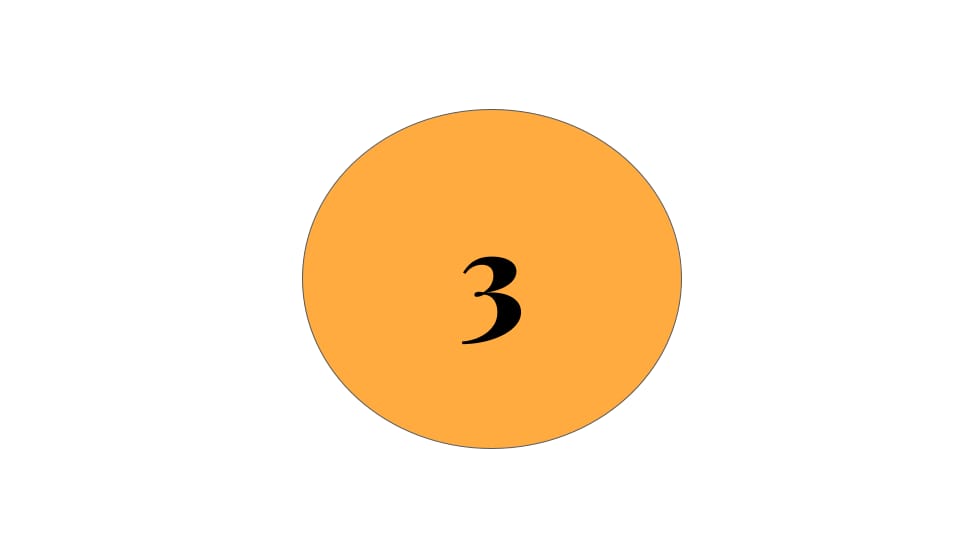
4 Signals from recent weeks

The tobacco product standard to prohibit the use of menthol as a characterizing flavor in cigarettes (RIN: 0910-AI60) will be delayed as noted in a statement by HHS Secretary Xavier Bercerra. This rule has garnered historic attention and the public comment period has yielded an immense amount of feedback, including from various elements of the civil rights and criminal justice movement. It’s clear that there are still more conversations to have, and that will take significantly more time.

Altria and Philip Morris International reported Q1 2024 earnings. PMI’s ZYN continues to grow in the oral smoke-free segment (ex snuff, snuff leaf and U.S. chew) with a category share of 74% in the U.S. Altria reported a decline of 10% in its smokeable products segment in Q1 24. Atria also sent a letter to Brian King, Director of CTP, outlining 10 recommended enforcement actions against illicit e-vapor products.
FDA CTP continued to issue civil monetary penalties to 20 brick-and-mortar and 2 online retailers. This trend represents a continued acceleration in enforcement actions, now including follow-up inspections to previous violations. FDA also issued several MDOs to manufacturers.

A new website was published by FDA CTP titled The Relative Risks of Tobacco Products which discusses the options available to adults who smoke cigarettes. As stated, Many people who use tobacco products have misperceptions about nicotine and the risks of various tobacco products. This website attempts to educate adult smokers on the actual risk profiles of different tobacco products.