- Smoke Signals: Navigating the Evolving Landscape of Nicotine and Tobacco Regulations
- Posts
- August 5, 2024 - The U.S. nicotine market is evolving
August 5, 2024 - The U.S. nicotine market is evolving
Issue #15 - Introducing a new nicotine regulatory intelligence dashboard
Nicotine Regulatory Intelligence Dashboard created
Philip Morris International, British American Tobacco and Altria reported Second-Quarter 2024 earnings
FDA released a status report on the remaining covered PMTA applications in FDA CTP review
As FDA CTP transitions from evaluating covered ENDS PMTA applications to other PMTAs in their review queue, including nicotine pouches, it will be interesting to see how CTP views these novel products within the context of APPH. ENDS PMTA evaluations focus on aerosol chemistry and device engineering among other disciplines while nicotine pouch evaluations center around chemical composition of the pouch materials, nicotine delivery profiles and oral health.
The increasing number of scientific publications and clinical trials focused on oral nicotine pouches appears to reflect this transition as well.
The ✅ PMTA Checklist I created below is a high-level draft that is product-agnostic. Going forward, as nicotine product portfolios become more diverse, having regulatory tools that serve multiple product categories might become more valuable.

New Nicotine Regulatory Intelligence Dashboard
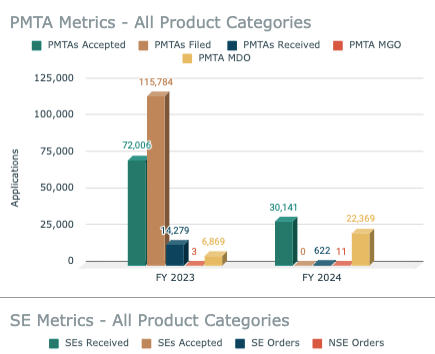
I created a nicotine regulatory intelligence dashboard to monitor developments across the FDA Center for Tobacco Product (CTP) landscape. I wanted to be able to see a more holistic look at various elements of CTP application review progress, policy and enforcement. It currently includes:
CTP Warning Letters by Month
CTP Director Meetings by Month
PMTA Metrics - All Product Categories
SE Metrics - All Product Categories
Exemption Metrics - All Product Categories
MGOs/MDOs - Last 90 Days
FOIAs Submitted vs Received - Last 30 Days
Tobacco Retailer Underage Sales Violations by State
PMTA Metrics - Breakout by Product Category

British American Tobacco, Altria and Philip Morris International Reported Second Quarter 2024 Earnings
New Categories:
Significant growth in non-combustible products.
Added 1.4 million new consumers, reaching a total of 26.4 million.
New Categories now account for 18% of group revenue.
Key Takeaways:
Altria stated they are beginning to see illicit activity across multiple tobacco categories, including nicotine pouches and cigarettes.
Second Quarter 2024 - Smokeable products segment reported domestic cigarette shipment volume decreased 13.0%, primarily driven by the industry’s decline rate
Smoke-Free Products:
Smoke-free products accounted for 38.1% of total net revenues, up by 2.7 percentage points year-over-year.
Total IQOS users reached 30.8 million, with 22.1 million fully switching to IQOS.
The FDA has acted upon 185 out of 186 manufacturers who came into compliance with new regulatory requirements related to tobacco products.
The plaintiffs moved to modify the remedial order to request regular status reports estimating the completion of the review of certain tobacco products.
The court granted the modification, requiring the FDA to submit status reports every 90 days regarding specific tobacco products.
Deep-Dive: PMTA Checklists
"Is there a PMTA checklist?" - The premarket tobacco product application process is by no means simply a checklist exercise but I get this question quite often. Checklists are intuitive and show demonstrable progress so I thought I would provide an example here. It’s possible to go 3 levels deeper for each of these sections.
PMTA Checklist
1. Administrative Requirements
FDA Forms:
☐ FDA Form 4057 (New PMTA)
☐ FDA Form 4057b (Product Grouping Spreadsheet)
☐ FDA Form 4057a (Amendments or General Correspondence)
Application Format:
☐ Ensure compliance with format requirements as per 21 CFR 1114.7(b) for standard PMTA.
☐ Table of Contents provided.
Right of Reference:
☐ Include right of reference for applications referencing Tobacco Product Master Files (TPMFs).
Translations:
☐ Certified English translations of any non-English documents.
☐ Certification statement by an authorized representative confirming translations are complete and accurate.
☐ Statement of qualifications of the translator.
2. Product Description and Labeling
☐ Detailed description of the tobacco product, including:
☐ Product name
☐ Product category and subcategory
☐ Product components and ingredients
☐ Manufacturing processes
☐ Labeling and marketing information:
☐ Product labels
☐ Marketing materials and advertisements
☐ Packaging specifications
3. Environmental Assessment (EA)
☐ Product-specific Environmental Assessment, including:
☐ Impact of product use
☐ Impact of product disposal
☐ Any mitigation measures for potential environmental impacts
4. Health Risk Assessment
☐ Nonclinical studies:
☐ Toxicology data
☐ In vitro and in vivo studies
☐ Genotoxicity studies
☐ Clinical studies:
☐ Human clinical trials
☐ Pharmacokinetics and pharmacodynamics data
☐ Abuse liability studies
☐ Literature Review:
☐ Comprehensive review of existing scientific literature related to the product and its constituents.
5. Behavioral and Perception Studies
☐ Studies on consumer perception and use patterns:
☐ Surveys and focus groups
☐ Behavioral studies on initiation, cessation, and dual use
☐ Marketing studies
6. Product Chemistry and Stability
☐ Detailed product chemistry:
☐ Chemical composition
☐ Nicotine content and delivery
☐ Harmful and potentially harmful constituents (HPHCs)
☐ Stability testing data:
☐ Shelf-life studies
☐ Storage conditions and stability over time
7. Manufacturing Information
☐ Comprehensive manufacturing process description:
☐ Facilities and equipment used
☐ Quality control measures
☐ Batch production records
☐ Good Manufacturing Practices (GMP) compliance:
☐ GMP certification or compliance statement
☐ Quality assurance protocols
8. Postmarket Surveillance and Reporting
☐ Plan for postmarket surveillance:
☐ Methods for monitoring adverse events
☐ Procedures for reporting to FDA
☐ Consumer complaint management
☐ Risk management plan:
☐ Strategies for mitigating identified risks
☐ Plans for addressing emerging risks
9. Executive Summary
☐ Comprehensive executive summary of the PMTA, including:
☐ Overview of the product and its intended use
☐ Summary of key findings from health risk assessments, behavioral studies, and manufacturing information
☐ Justification for marketing authorization
Acceptance Criteria (Based on CTP Reviewers’ Guidelines)
Completeness and Organization:
☐ Ensure all required sections and documents are included.
☐ Clearly labeled and organized application with a table of contents.
Regulatory Compliance:
☐ Compliance with format and content requirements as per 21 CFR 1114.
☐ Inclusion of all required FDA forms.
Scientific and Technical Data:
☐ Sufficient scientific and technical data to support safety and efficacy.
☐ Comprehensive health risk assessments and environmental assessments.
Right of Reference and Intellectual Property:
☐ Appropriate rights of reference for any referenced TPMFs or third-party data.
Translation and Certification:
☐ Complete and accurate translations with proper certification.
Labeling and Marketing Consistency:
☐ Consistency between product labeling, marketing materials, and the application content.
Chatbots
🤖 PMTA MGO Chatbot - This large language model (LLM) response agent was trained on several PMTA marketing granted orders and their respective technical project lead memos issued by FDA CTP. 8/5 updates include the VUSE Alto tobacco-flavored MGOs from July 18, 2024.
I am making available a strategic analysis of CTP Application Job Aids. This resource provides a step-by-step analysis of the job aids provided to FDA CTP reviewers assigned to premarket tobacco product applications (PTMA), exemption request (EX) and substantial equivalence (SE) applications submitted under sections 910(a), 905(j)(A)(3) or 905(j), respectively, of the Federal Food, Drug, and Cosmetic Act (FD&C Act). The document includes the strategic analysis as well as the job aids.


