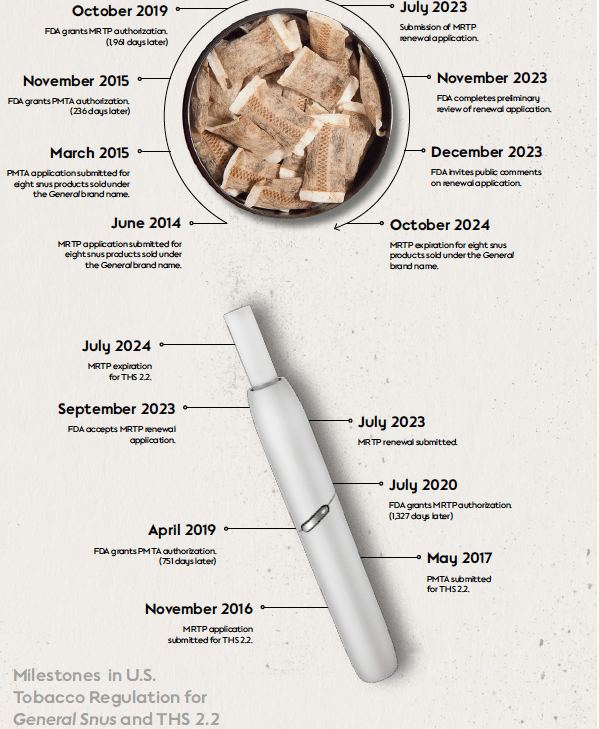
- Smoke Signals: Navigating the Evolving Landscape of Nicotine and Tobacco Regulations
- Posts
- FDA FOIA logs and PMTA metrics updates - March 4, 2024
FDA FOIA logs and PMTA metrics updates - March 4, 2024
Issue #4
Time is the scarcest resource, and unless it is managed, nothing else can be managed.
Freedom of Information Act (FOIA) logs were updated for January and February 2024 and I compiled the relevant nicotine and tobacco-related items on my website. I’ve also written previously on strategies to effectively use FOIA to build a regulatory intelligence program.
It is apparent that FOIA requests are becoming a singularly available (and desperate) part of the regulatory intelligence collection process for manufacturers submitting applications to CTP. This is likely due to the lack of transparency in releasing guidance documents/reviewer memos through other distribution channels and the ongoing litigation for various ENDS product MDOs preventing CTP from acting. This application information is vital for manufacturers to understand the review processes being deployed to evaluate applications at CTP.
Freedom of Information Act (FOIA) requests can be an important component of a comprehensive regulatory intelligence program. Requests can be made to FDA or any other government agency. It is important to craft the request in a way that is specific to a particular data set and that the request is time-bound. Including relevant submission tracking numbers (STNs) can also be immensely helpful in achieving a successful outcome.
By my estimation there were over 30 nicotine and tobacco-relevant new FOIAs submitted and at least 13 closed-out FOIAs in January and February 2024. Several requests were made for the SMOK MDOs issued by CTP on January 16, 2024 as well as the blu PLUS+ MDOs issued on January 22, 2024. Multiple requests for the same information should increase the likelihood of success with these requests.
Among the closed-out FOIAs it seems the FOIA process is becoming slower. The volume of requests to the FOIA Office has increased over the last year. A response time of 6 months used to be the average but some requests from 2022 are recently being fulfilled, indicating perhaps a year of backlog. At some point in the future I will share the FOIAs I’ve received.
PMTA
 | Vapor unit c-store sales were down in 2023 but dollar sales were up. There still seems to be pricing power in the vapor space. The Food and Drug Administration (FDA) has approved fewer than 25 tobacco-flavored e-cigarettes and vaping devices for legal retail sales. By the end of 2023, FDA still hadn’t issued decisions on major brands, including JUUL and some Vuse and Alto products. However, by the end of January, the FDA confirmed that it has issued more than 440 letters warning brick-and-mortar stores along with online retailers to pull unauthorized products, including Esco Bars. Anne Baye Ericksen |
Altria and Philip Morris International each presented at the CAGNY conference in February 2024. It was a similar story to each company’s recent annual report guidance, reinforcing their respective smoke-free product opportunities.
 |
|
 |
|
Policy
 | Actions on applications in 2023 – including Vuse Solo, Vuse Alto, Vuse Vibe, Vuse Ciro, and myblu – together with recent MDO decisions for blu PLUS+, Suorin, SMOK, Bidi, and blu Disposable and myBlu e-cigarette products, means that the center has now acted on 94 percent of the higher market share applications it reports on to a federal court. (February 22, 2024) |
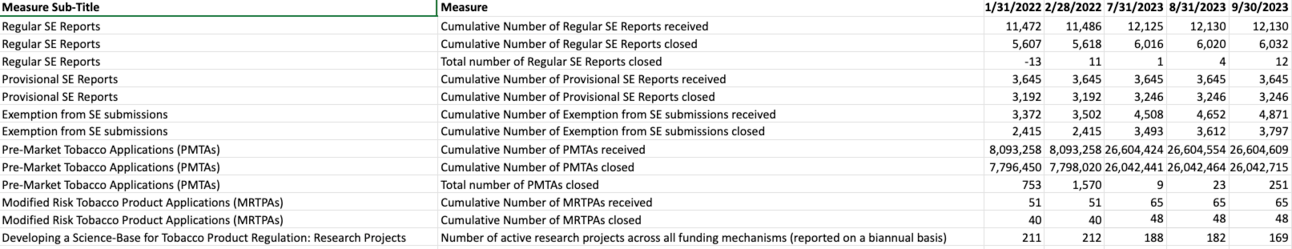
FDA updates its FDA TRACK Dashboard biannually which is the source of this data. As of 2024 Q1 the number of active funding mechanisms for research stands at 169. The number of research projects has dropped from 211 in January 2022. This could be for a number of reasons including new TCORS 3.0 centers being established. It is interesting to see in this table the cumulative number of SE Reports closed and cumulative number of PMTAs closed rapidly declining.
Through December 31, 2023:
➢Cumulative Number of PMTAs received - 26,606,552
➢Cumulative Number of PMTAs closed - 26,045,440
➢Cumulative Number of MRTPAs received - 83
➢Cumulative Number of MRTPAs closed - 48
➢Active research projects across all funding mechanisms - 187
 | On Feb. 26, FDA announced the filing of complaints for civil money penalties (CMPs) against 20 brick and mortar retailers for the sale of unauthorized Elf Bar e-cigarettes. These violations were each over $20K per retailer. |
Opinion
 | The FDA’s Center for Tobacco Products Ignores the Science of Tobacco Harm Reduction (February 28, 2024) The CTP should act now to revise its processes and procedures and open the ENDS marketplace to products with varied characteristics (e.g., format, flavor, nicotine level) so that those interested in alternative nicotine products can access them. A diverse range of ENDS products available to those who smoke and want to quit is critical to reducing the health burdens associated with smoking. Continued delay by the CTP will only lead to more unnecessary deaths and disease in the United States. Jeffrey Smith |
Recent Publications of Interest
The two recent studies presented below represent the evolving nature of nicotine and tobacco research, separating the health impacts of smoking combustible cigarettes from the those of nicotine and nicotine-vaping specifically. There are hundreds of these studies published every month. ENDS Literature PubMed search here.
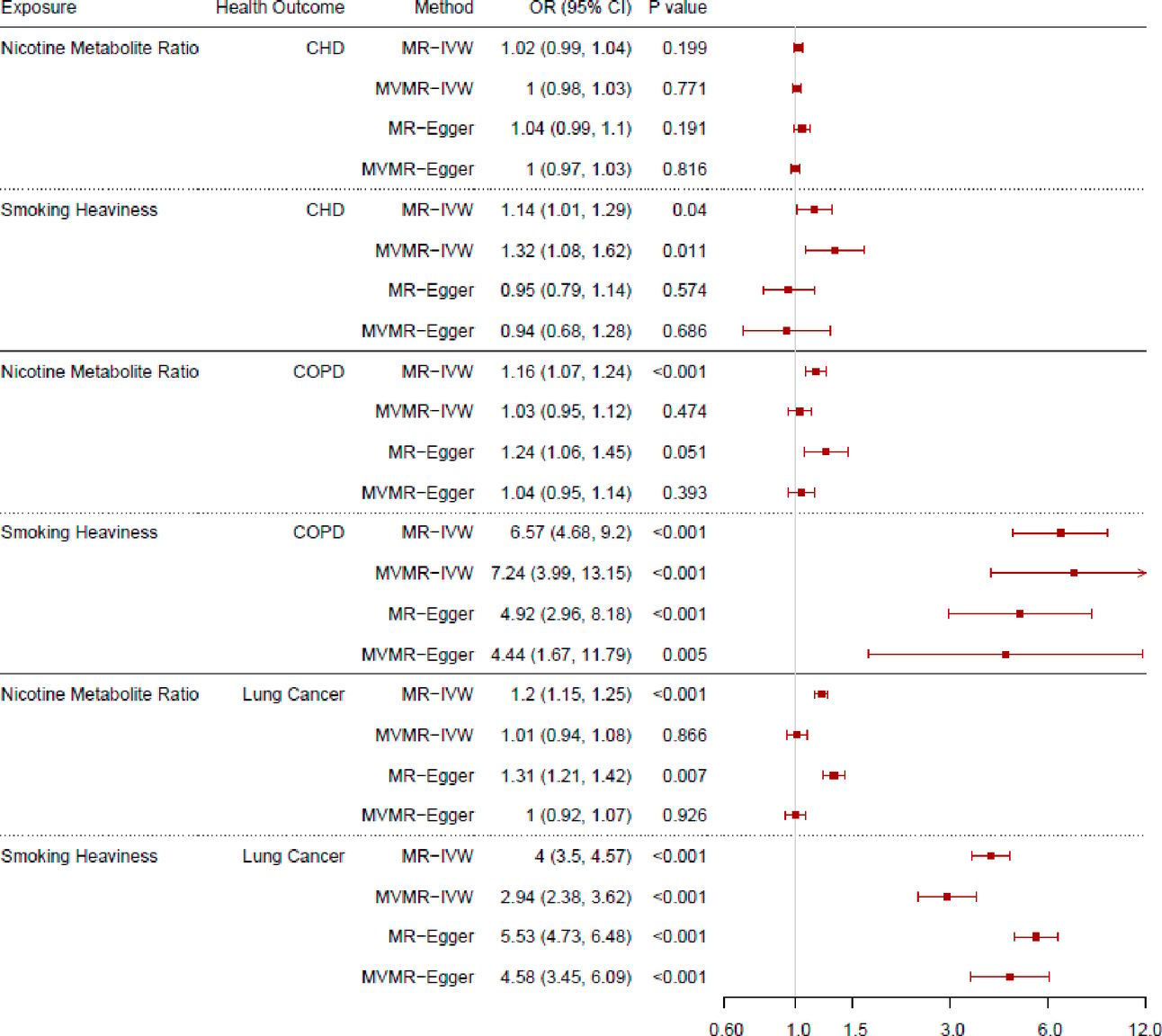
Khouja JN, Sanderson E, Wootton RE, Taylor AE, Church BA, et al. (2024) Estimating the health impact of nicotine exposure by dissecting the effects of nicotine versus non-nicotine constituents of tobacco smoke: A multivariable Mendelian randomisation study. PLOS Genetics 20(2): e1011157. | Partial Abstract: Although we know that smoking tobacco negatively impacts health, we know relatively little about whether nicotine plays a role in causing poor health outcomes. When used for short periods of time, nicotine appears to have little impact on health. However, until recently, nicotine has rarely been used for long periods without accompanying exposure to tobacco smoke, so it is hard to disentangle the effects of regular nicotine use from the effects of tobacco smoke exposure. |
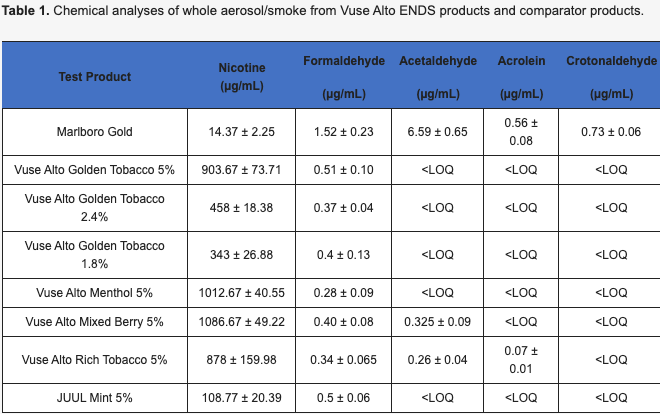
Keyser BM, Leverette R, Wertman J, Shutsky T, McRae R, Szeliga K, Makena P, Jordan K. Evaluation of Cytotoxicity and Oxidative Stress of Whole Aerosol from Vuse Alto ENDS Products. Toxics. 2024; 12(2):129. | Partial Abstract: In the present study, a whole aerosol (WA) system was used to expose lung epithelial cultures (2D and 3D) to determine the potential of six Vuse Alto ENDS products that varied in nicotine content (1.8%, 2.4%, and 5%) and flavors (Golden Tobacco, Rich Tobacco, Menthol, and Mixed Berry), along with a marketed ENDS and a marked cigarette comparator to induce cytotoxicity and oxidative stress. |
Clinical Trials
The clinical trial space has been evolving over the last year for ENDS products. There have been less registered trials for what CTP has determined to be “covered applications” with the highest tracked market share. ENDS Clinical Trial Feed here.
 | This is a pilot study on the impact of switching from cigarettes to Electronic Cigarettes (EC) on disease-related clinical symptoms and biomarkers of harm in smokers with preexisting Chronic Obstructive Pulmonary Disease (COPD). The researchers hypothesize that the smokers who switch to EC completely or significantly will experience reduced COPD symptoms, risks of exacerbations, and decreased levels of oxidative stress and inflammation. |
Arm | Sponsor | Estimated Study Completion Date |
|---|---|---|
NJOY e-cigarette 5% | Penn State Health Milton S. Hershey Medical Center | November 2024 |
Resource Links
J.E. Dice Regulatory Solutions weekly newsletters - These are AI-generated weekly data dumps, probably 100 articles per week in trade press and lit publications. |