- Smoke Signals: Navigating the Evolving Landscape of Nicotine and Tobacco Regulations
- Posts
- July 8, 2024 - U.S. Supreme Court Rules on Vaping
July 8, 2024 - U.S. Supreme Court Rules on Vaping
Issue #13 - TPMP risk assessment
TPSAC meeting for General Snus MRTPA renewal was held on June 26, 2024.
The Senate Judiciary Committee requested information from DOJ and FDA on the recently announced ENDS Enforcement Task Force.
Altria and Reynolds announced submission of Premarket Tobacco Product Applications. Altria for several on! PLUS nicotine pouch products and Reynolds for their VUSE Alto Plus age-gated ENDS device.
The Supreme Court ruling in LOPER BRIGHT ENTERPRISES ET AL v. RAIMONDO ET AL overruled the Chevron doctrine, which required courts to defer to an agency's interpretation of a statute if the statute was ambiguous. In other SCOTUS news, a petition for a writ of certiorari was granted in the FOOD AND DRUG ADMINISTRATION v. WAGES AND WHITE LION INVESTMENTS, L.L.C., DBA TRIION DISTRIBUTION, ET. AL. case.


The Tobacco Product Scientific Advisory Committee (TPSAC) held a hearing on June 26, 2024 to discuss the renewal of Swedish Match’s General Snus Modified Risk Tobacco Product Application (MRTPA), 5 years after the initial risk modification order was granted.
Swedish Match's MRTPAs for General Snus included the following proposed modified risk claim:
"Using General Snus instead of cigarettes puts you at a lower risk of mouth cancer, heart disease, lung cancer, stroke, emphysema, and chronic bronchitis."
From the Swedish Match Briefing Materials - June 26, 2024


Richard Durbin (D-IL) sent a letter to to Deputy Assistant Attorney General of the Department of Justice’s (DOJ’s) Consumer Protection Branch, Arun Rao, and the U.S. Food and Drug Administration (FDA) Center for Tobacco Products Director, Dr. Brian King, requesting more information about the recently announced interagency task force to combat the illicit sale and distribution of unauthorized e-cigarettes. Rao and King testified recently at Judiciary Committee hearing on e-cigarettes. Responses to these questions were requested by July 26, 2024:
How will the task force prioritize enforcement actions between and among manufacturers, distributors, and retailers?
Will the unlawful sale or distribution of unauthorized e-cigarettes, including those with pending PMTAs, be within the scope of enforcement for the task force?
How will the task force augment FDA's issuance of warning letters and civil monetary penalties? Specifically, will the task force provide resources to increase the number of inspections or shorten the timeline for re-inspections to assess compliance?
There have been a very limited number of injunctions or seizures related to the unlawful distribution and sale of unauthorized e-cigarettes. How will the task force enhance the use of these enforcement actions?
What new civil and criminal authorities, aside from violations of Sections 902 and 903 of the Federal Food, Drug, and Cosmetic Act, does the task force anticipate using? Do you plan to request additional authorities from Congress for this purpose?

Altria announced the submission of PMTAs to the U.S. Food and Drug Administration (FDA) for its innovative on! PLUS oral nicotine pouch products (three flavor varieties, each with three different nicotine strength options). The PMTAs were submitted by Altria’s wholly owned subsidiary Helix Innovations LLC (Helix).
Reynolds completed PMTA submissions for age-gated VUSE Pro e-cigarette platform. The electronic nicotine delivery system (ENDS) device platform connects to a mobile application that verifies the consumer’s age through a third-party provider prior to unlocking for use.


The Supreme Court ruling in LOPER BRIGHT ENTERPRISES ET AL v. RAIMONDO ET AL overruled the Chevron doctrine, which required courts to defer to an agency's interpretation of a statute if the statute was ambiguous. The ruling clarified that courts must exercise independent judgment when deciding whether an agency has acted within its statutory authority.
For businesses, this ruling has significant implications. It means that courts will no longer automatically defer to an agency's interpretation of a statute when it is ambiguous. Instead, courts will independently assess whether an agency has acted within its statutory authority. This could lead to more scrutiny of agency actions and potentially more legal challenges to agency decisions. Businesses may need to be more proactive in understanding and challenging agency actions that affect them, as courts will now play a more commanding role in reviewing regulatory statutes.
The implications for flavored vaping are discussed in this clip @ 2:40
The Supreme Court overturning the Chevron Doctrine, which could undercut federal agencies like the FDA. Former Commissioner @ScottGottliebMD explains the impact:
— Squawk Box (@SquawkCNBC)
11:07 AM • Jul 1, 2024
Relatedly, the U.S. Supreme Court granted a petition for a writ of certiorari in the FOOD AND DRUG ADMINISTRATION v. WAGES AND WHITE LION INVESTMENTS, L.L.C., DBA TRIION DISTRIBUTION, ET. AL.
Here are the key points:
The FDA denied marketing authorization for flavored e-cigarette products made by Wages and White Lion Investments (Triton Distribution) and Vapetasia LLC.
The U.S. Court of Appeals for the Fifth Circuit set aside the FDA's denial orders as arbitrary and capricious.
The FDA is now petitioning the Supreme Court to review and reverse the Fifth Circuit's decision.
The main issues in contention are:
Whether the FDA unfairly surprised manufacturers by changing evidentiary standards for applications
Whether the FDA's failure to consider marketing plans was harmless error
Whether the FDA arbitrarily ignored distinctions between different types of e-cigarette devices
Whether the FDA has effectively imposed a categorical ban on flavored e-cigarettes
The petition argues that the Fifth Circuit's decision conflicts with rulings from seven other circuit courts on similar issues.
The FDA contends that the Fifth Circuit's decision undermines efforts to prevent youth e-cigarette use and frustrates the goals of the Tobacco Control Act.
The Solicitor General is requesting that the Supreme Court grant the petition for certiorari to resolve the circuit split and address the important public health issues at stake.
This petition represents an attempt by the FDA to have the Supreme Court review and potentially overturn the Fifth Circuit's decision that was unfavorable to the agency's regulation of flavored e-cigarette products.
Deep Dive
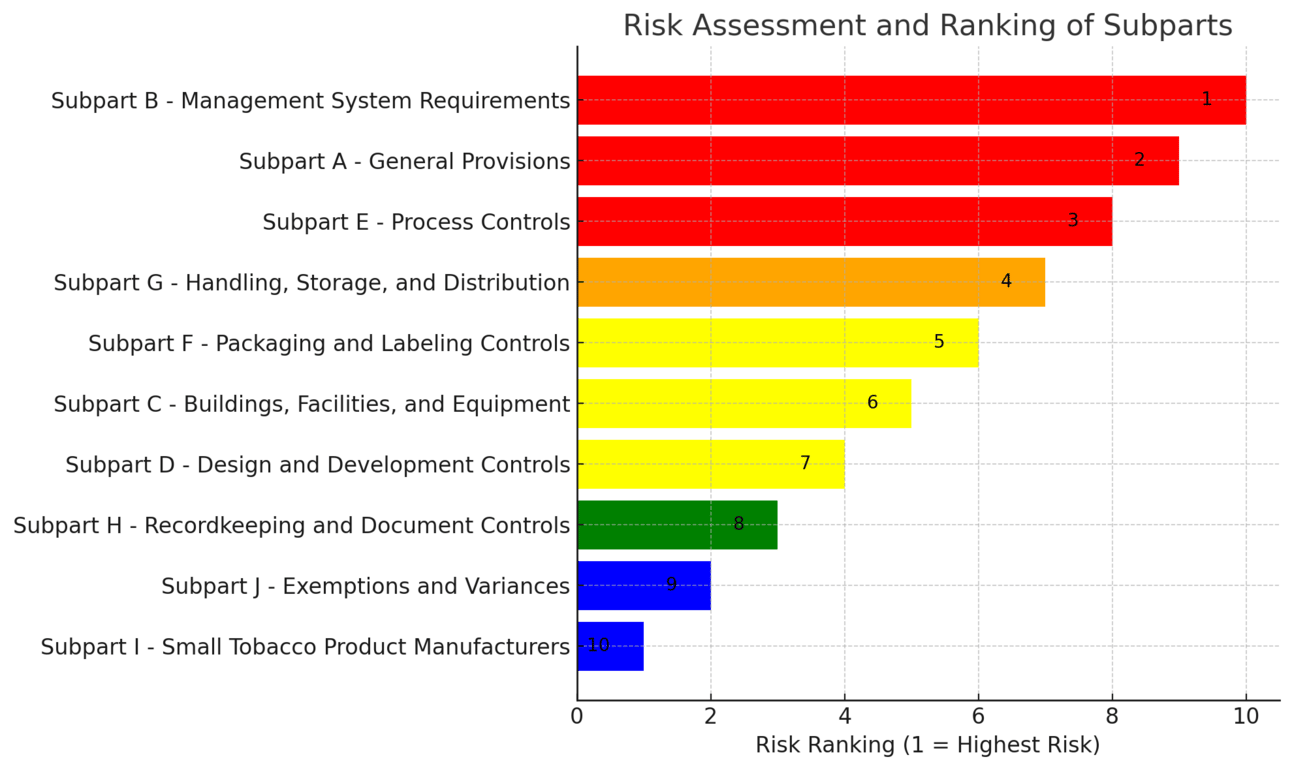
Tobacco Product Manufacturing Practice (TPMP) Risk Assessment and Ranking
To perform a risk assessment for each subpart of the TPMP, we'll consider factors such as the complexity of compliance, potential impact of non-compliance, resource demands, and likelihood of regulatory changes or challenges. Each subpart will be evaluated on these criteria and ranked from highest to lowest risk.
Subpart B - Management System Requirements
Challenges: Establishing an effective organizational structure, handling complaints, and implementing CAPA are critical to ensuring overall compliance. Failures here can have significant regulatory and operational consequences.
Resource Allocations: High due to the need for trained personnel, systems, and extensive documentation.
Risk Level: High
Subpart A - General Provisions
Challenges: Interpreting scope and definitions accurately is foundational. Misunderstandings here can lead to widespread non-compliance.
Resource Allocations: Legal and compliance expertise, continuous updates.
Risk Level: High
Subpart E - Process Controls
Challenges: Purchasing controls, acceptance activities, and handling nonconforming products directly affect product quality and safety.
Resource Allocations: Significant investment in quality assurance and tracking systems.
Risk Level: High
Subpart G - Handling, Storage, and Distribution
Challenges: Ensuring product integrity and traceability throughout the supply chain is crucial. Failures can lead to contamination and regulatory penalties.
Resource Allocations: High due to the need for robust storage facilities, distribution systems, and tracking technologies.
Risk Level: Medium-High
Subpart F - Packaging and Labeling Controls
Challenges: Compliance with packaging and labeling regulations is critical to prevent mix-ups and ensure accurate product information.
Resource Allocations: Moderate, involving approval processes, technology solutions, and training.
Risk Level: Medium
Subpart C - Buildings, Facilities, and Equipment
Challenges: Ensuring suitable facilities and equipment to prevent contamination is essential.
Resource Allocations: Investment in infrastructure and regular maintenance.
Risk Level: Medium
Subpart D - Design and Development Controls
Challenges: Controlling design and development to manage risks and ensure product quality is complex but critical.
Resource Allocations: Teams with expertise and tools for verification and validation.
Risk Level: Medium
Subpart H - Recordkeeping and Document Controls
Challenges: Accurate recordkeeping and document control are fundamental to compliance but are more straightforward to implement.
Resource Allocations: Recordkeeping systems, trained personnel, and audits.
Risk Level: Medium-Low
Subpart J - Exemptions and Variances
Challenges: Petitioning for exemptions and variances is a specialized, albeit less frequent, task.
Resource Allocations: Legal and regulatory resources, documentation.
Risk Level: Low
Subpart I - Small Tobacco Product Manufacturers
Challenges: Smaller manufacturers have delayed compliance but face similar requirements, often with fewer resources.
Resource Allocations: Planning and incremental implementation.
Risk Level: Low
Ranking of Subparts by level of risk to implement
Subpart B - Management System Requirements
Subpart A - General Provisions
Subpart E - Process Controls
Subpart G - Handling, Storage, and Distribution
Subpart F - Packaging and Labeling Controls
Subpart C - Buildings, Facilities, and Equipment
Subpart D - Design and Development Controls
Subpart H - Recordkeeping and Document Controls
Subpart J - Exemptions and Variances
Subpart I - Small Tobacco Product Manufacturers
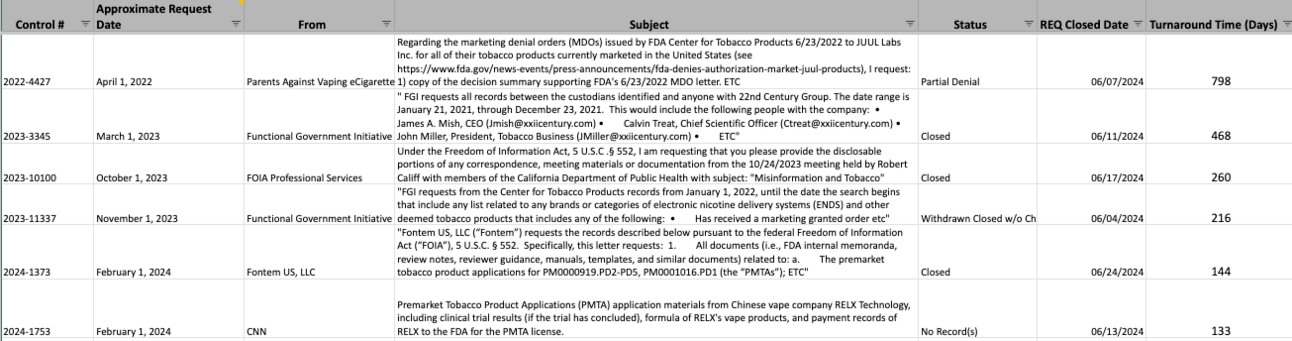
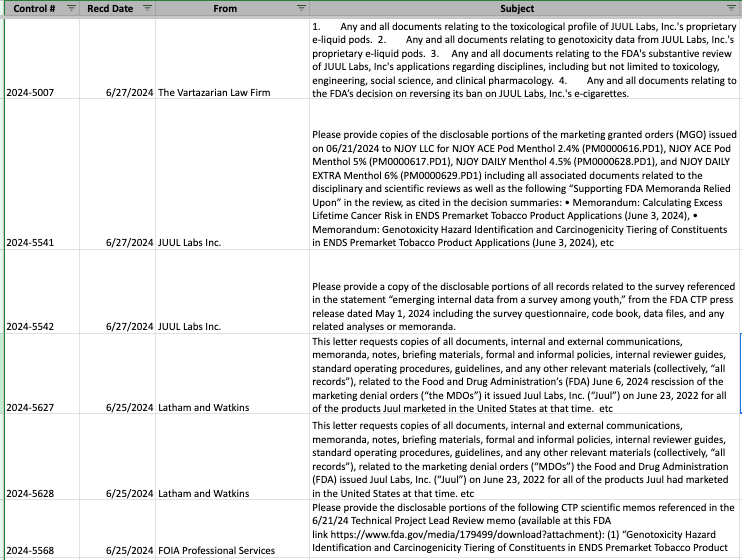
Freedom of Information Act Updates for June 2024
Closed requests:
New requests:
Chatbots
PMTA MGO Chatbot - This large language model (LLM) response agent was trained on several PMTA marketing granted orders and their respective technical project lead memos issued by FDA CTP. 7/8 updates include the NJOY ACE and DAILY Menthol PMTA MGOs.
I am making available a strategic analysis of CTP Application Job Aids. This resource provides a step-by-step analysis of the job aids provided to FDA CTP reviewers assigned to premarket tobacco product applications (PTMA), exemption request (EX) and substantial equivalence (SE) applications submitted under sections 910(a), 905(j)(A)(3) or 905(j), respectively, of the Federal Food, Drug, and Cosmetic Act (FD&C Act). The document includes the strategic analysis as well as the job aids.