- Smoke Signals: Navigating the Evolving Landscape of Nicotine and Tobacco Regulations
- Posts
- July 22, 2024 - VUSE Alto Tobacco MGOs⌛️
July 22, 2024 - VUSE Alto Tobacco MGOs⌛️
Issue #14 - Overview of the Chemistry of ENDS and E-Liquids
FDA CTP announces a new updated submission portal for new tobacco product applications in 2025
VUSE Alto Tobacco flavor marketing granted orders issued after almost 4 years in review
Updates to tobacco retailer underage compliance check data
Deep Dive - Briefing on the chemistry of electronic nicotine delivery systems (ENDS) and e-liquids.


Beginning 7/16, public may provide comments until 9/14.
The improvements are intended to:
Streamline electronic submission into one system for substantial equivalence (SE) reports and premarket tobacco product applications (PMTAs), amendments to previous submissions, and submission of general correspondence;
Introduce a more efficient submission process by eliminating the need for multiple tools, including combining PDF-editing software, FDA’s eSubmitter Desktop Tool, and FDA’s CTP Portal Web application in one place;
This tool will provide tools to expedite data entry, guide applicants to relevant sections, and verify that all required data have been provided by the applicant.


R.J. Reynolds Vapor Company received U.S. FDA marketing authorization for six Vuse Alto tobacco-flavored pods, which are sealed, pre-filled, and non-refillable and a Vuse Alto Power Device
Vuse Alto Pod Golden Tobacco 5%
Vuse Alto Pod Rich Tobacco 5%
Vuse Alto Pod Golden Tobacco 2.4%
Vuse Alto Pod Rich Tobacco 2.4%
Vuse Alto Pod Golden Tobacco 1.8%
Vuse Alto Pod Rich Tobacco 1.8%
These applications were submitted in September 2020 and authorized almost 4 years later in July 2024. The VUSE Alto Menthol and Mixed Berry Pods were denied in October 2023.
Summary of the technical project lead memo:
Product Characterization:
The e-liquids contain nicotine salt formulations that may be easier to inhale at high amounts of nicotine, facilitating initiation and use of ENDS with high nicotine concentrations (p. 7).
Abuse Liability:
The abuse liability of the new products is lower than combusted cigarettes and is similar to, or lower than, that of other ENDS (p. 27).
Toxicant Exposure:
The overall toxicological risk to users of the new products is lower compared to cigarettes due to significant reductions in aerosol HPHCs (Harmful and Potentially Harmful Constituents) of the new products compared to cigarettes (p. 27).
Population and Public Health:
The applicant demonstrated that current adult smokers are particularly interested in the new products to assist in intended switching, and these products have the potential to benefit that group as compared to continued exclusive cigarette use (p. 4, 27).
Statutory Requirements:
FDA's evaluation determined that there are adequate process controls and quality assurance procedures to help ensure both the device and e-liquids are manufactured consistently (p. 27).
Youth Use Considerations:
The review discusses the potential for youth use of the electronic nicotine delivery system (ENDS) by highlighting that the applicant did not provide direct data on youth (p. 29).
Toxicology:
The new products and the ENDS comparison product, Vuse Alto Original 5%, aerosols demonstrated no cytotoxicity (neutral red uptake [NRU] assay) at the concentrations and under the conditions tested (p. 47).
The applicant provided supporting data from published in vitro and in vivo toxicology literature on respiratory effects, carcinogenicity, cardiovascular effects, mutagenicity and genotoxicity effects, reproductive/developmental effects, immunotoxicity, neurotoxicity effects, and other systemic effects (p. 47).
Comparative Analysis:
The applicant tested and provided mainstream aerosol HPHC yields from Vuse Alto (Original flavor pod, 5% w/w nicotine, Menthol, 5% w/w nicotine), Blu PLUS+ (Classic Tobacco, 2.4% w/w nicotine, Menthol, 2.4% w/w nicotine), and NJOY Ace (Classic Tobacco, 5% w/w nicotine, Mint 5% w/w nicotine) for comparison (p. 9).
The rationale for selecting combustible cigarettes and other commercially available ENDS as comparison products is reasonable given their general product composition and design (p. 9).
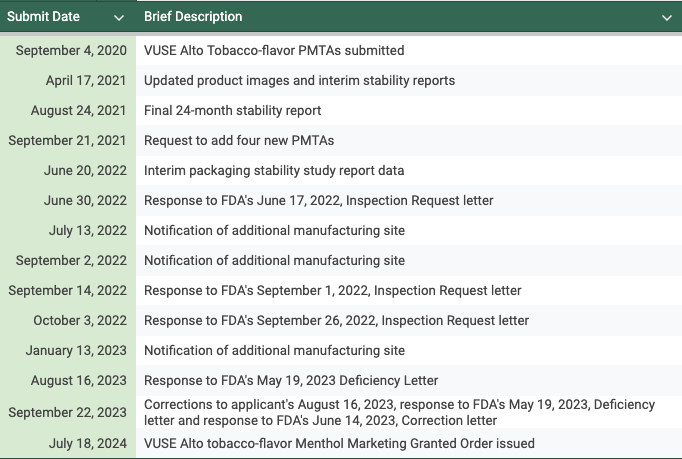
Regulatory Timeline for VUSE Alto Tobacco PMTA review
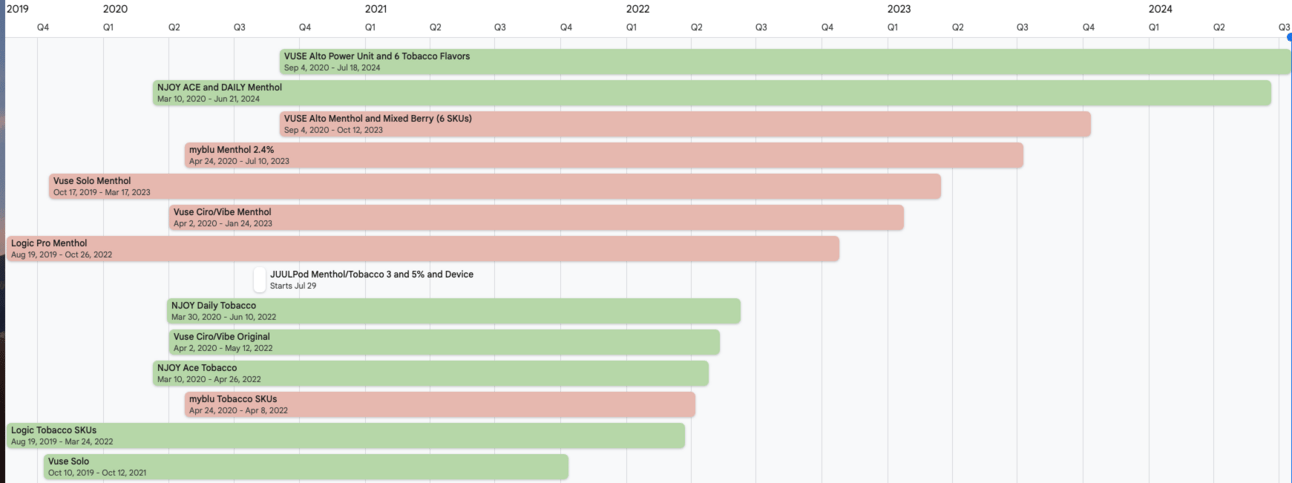
MDO/MGO Timelines for various ENDS products as of 7/19/24
CTP’s Office of Compliance and Enforcement (OCE) conducts brick-and-mortar tobacco retailer inspections for underage tobacco product purchase across the U.S. This chart shows the difference between Fiscal Year 2023 (October 2022 to September 2023) to Fiscal Year 2024 (October 2023 to September 2024). Year-to-date totals indicate a drop in the overall violation rate from 16.3% to 14.7%.
Deep-Dive: Briefing on the Chemistry of Electronic Nicotine Delivery Systems (ENDS) and E-Liquids
Outline
Introduction
Definition and Overview of ENDS
Purpose of the Briefing
Historical Background
Evolution of ENDS
Key Milestones in ENDS Development
Chemical Composition of E-Liquids
Primary Ingredients
Nicotine Forms
Flavoring Agents
Additives and Preservatives
Chemical Reactions and Byproducts
Aerosol Formation
Thermal Decomposition
Harmful and Potentially Harmful Constituents (HPHCs)
Analytical Methods for Chemical Characterization
Chromatography Techniques
Spectroscopy Methods
Mass Spectrometry
Other Analytical Techniques
Toxicological Considerations
Acute and Chronic Toxicity
Carcinogenicity
Respiratory and Cardiovascular Effects
Regulatory Standards and Compliance
FDA Guidelines
International Standards
Quality Control and Assurance
Conclusion
Summary of Key Points
Future Directions in ENDS Chemistry Research
1. Introduction
Definition and Overview of ENDS
Electronic Nicotine Delivery Systems (ENDS), commonly known as e-cigarettes, are devices that deliver nicotine through aerosolization of e-liquids. These systems are designed to mimic the experience of smoking traditional cigarettes but with reduced exposure to harmful constituents found in tobacco smoke.
Purpose of the Briefing
This briefing aims to provide a comprehensive overview of the chemistry involved in ENDS and e-liquids, focusing on their composition, chemical reactions, analytical methods, toxicological considerations, and regulatory standards.
2. Historical Background
Evolution of ENDS
The concept of ENDS dates back to the early 2000s, with the first commercially successful e-cigarette introduced by Hon Lik, a Chinese pharmacist, in 2003. Since then, ENDS have undergone significant technological advancements, leading to a variety of designs and functionalities.
Key Milestones in ENDS Development
2003: Introduction of the first modern e-cigarette
2007: Entry of e-cigarettes into the U.S. market
2010s: Emergence of pod-based systems and nicotine salts
2020: Implementation of stricter regulatory measures by the FDA
3. Chemical Composition of E-Liquids
Primary Ingredients
E-liquids typically contain four primary ingredients:
Propylene Glycol (PG): A solvent that produces a throat hit similar to smoking.
Vegetable Glycerin (VG): A thicker solvent that produces dense vapor clouds.
Nicotine: The addictive component, available in freebase or salt form.
Flavoring Agents: Various compounds that provide taste and aroma.
Nicotine Forms
Freebase Nicotine: The traditional form used in e-liquids, providing a harsher throat hit.
Nicotine Salts: A newer form that allows for higher nicotine concentrations with a smoother throat hit.
Flavoring Agents
Flavoring agents can be natural or synthetic and are used to enhance the sensory experience of vaping. Common flavors include fruit, menthol, and dessert flavors.
Additives and Preservatives
Additional compounds such as sweeteners, acids, and preservatives may be included to stabilize the e-liquid and enhance its properties.
4. Chemical Reactions and Byproducts
Aerosol Formation
When an e-liquid is heated by the coil in an ENDS device, it undergoes vaporization, forming an aerosol that is inhaled by the user.
Thermal Decomposition
At high temperatures, components of e-liquids can decompose, leading to the formation of potentially harmful byproducts such as formaldehyde, acetaldehyde, and acrolein.
Harmful and Potentially Harmful Constituents (HPHCs)
HPHCs identified in ENDS aerosols include:
Carbonyl compounds (e.g., formaldehyde, acetaldehyde)
Volatile organic compounds (VOCs)
Heavy metals (e.g., nickel, chromium)
Particulate matter
5. Analytical Methods for Chemical Characterization
Chromatography Techniques
Gas Chromatography (GC): Used for separating and analyzing volatile compounds.
Liquid Chromatography (LC): Suitable for non-volatile and thermally labile compounds.
Spectroscopy Methods
Nuclear Magnetic Resonance (NMR) Spectroscopy: Provides structural information about organic compounds.
Infrared (IR) Spectroscopy: Identifies functional groups in molecules.
Mass Spectrometry
Mass spectrometry is employed to determine the molecular weights and structures of compounds present in e-liquids and aerosols.
Other Analytical Techniques
High-Performance Liquid Chromatography (HPLC)
Inductively Coupled Plasma Mass Spectrometry (ICP-MS): For detecting heavy metals.
6. Toxicological Considerations
Acute and Chronic Toxicity
Studies have shown that while ENDS are less harmful than combustible cigarettes, they still pose risks of acute and chronic toxicity, particularly due to nicotine and other chemical constituents.
Carcinogenicity
Some byproducts of e-liquid decomposition, such as formaldehyde, are known carcinogens. However, the levels are generally lower than those found in tobacco smoke.
Respiratory and Cardiovascular Effects
ENDS use has been associated with respiratory issues such as bronchitis and asthma, as well as cardiovascular effects like increased heart rate and blood pressure.
7. Regulatory Standards and Compliance
FDA Guidelines
The FDA requires premarket tobacco product applications (PMTAs) for ENDS, which must demonstrate that the product is appropriate for the protection of public health.
International Standards
Various international bodies, such as the European Union, have established regulations for the manufacturing and marketing of ENDS.
Quality Control and Assurance
Manufacturers must implement stringent quality control measures to ensure the consistency and safety of their products. Tobacco Product Manufacturing Practices (TPMP) are being developed by FDA CTP to facilitate this work.
8. Conclusion
Summary of Key Points
ENDS and e-liquids are complex systems with various chemical components.
The primary ingredients include PG, VG, nicotine, and flavoring agents.
Chemical reactions during aerosolization can produce harmful byproducts.
Analytical methods are crucial for characterizing the chemical composition and ensuring product safety.
Regulatory standards are in place to protect public health.
Future Directions in ENDS Chemistry Research
Ongoing research aims to better understand the long-term health effects of ENDS use, improve the safety of e-liquid formulations, and develop more effective regulatory frameworks.
References
Food and Drug Administration. (2020). Enforcement Priorities for Electronic Nicotine Delivery Systems (ENDS) and Other Deemed Products on the Market Without Premarket Authorization (Revised)* (Guidance for Industry). Center for Tobacco Products, Food and Drug Administration, U.S. Department of Health and Human Services. Retrieved from https://www.fda.gov/media/133880/download
Villanti, A. C., Johnson, A. L., Glasser, A. M., et al. (2019). Association of Flavored Tobacco Use With Tobacco Initiation and Subsequent Use Among US Youth and Adults, 2013-2015. JAMA Network Open, 2(10), e1913804. https://doi.org/10.1001/jamanetworkopen.2019.13804
Voos, N., Smith, D., Kaiser, L., et al. (2019). Effect of e-cigarette flavors on nicotine delivery and puffing topography: Results from a randomized clinical trial of daily smokers. Psychopharmacology (Berl), 236(10), 3019-3027. https://doi.org/10.1007/s00213-019-05301-1
Chatbots
PMTA MGO Chatbot - This large language model (LLM) response agent was trained on several PMTA marketing granted orders and their respective technical project lead memos issued by FDA CTP. 7/22 updates include the VUSE Alto tobacco-flavored MGOs from July 18, 2024.
I am making available a strategic analysis of CTP Application Job Aids. This resource provides a step-by-step analysis of the job aids provided to FDA CTP reviewers assigned to premarket tobacco product applications (PTMA), exemption request (EX) and substantial equivalence (SE) applications submitted under sections 910(a), 905(j)(A)(3) or 905(j), respectively, of the Federal Food, Drug, and Cosmetic Act (FD&C Act). The document includes the strategic analysis as well as the job aids.