- Smoke Signals: Navigating the Evolving Landscape of Nicotine and Tobacco Regulations
- Posts
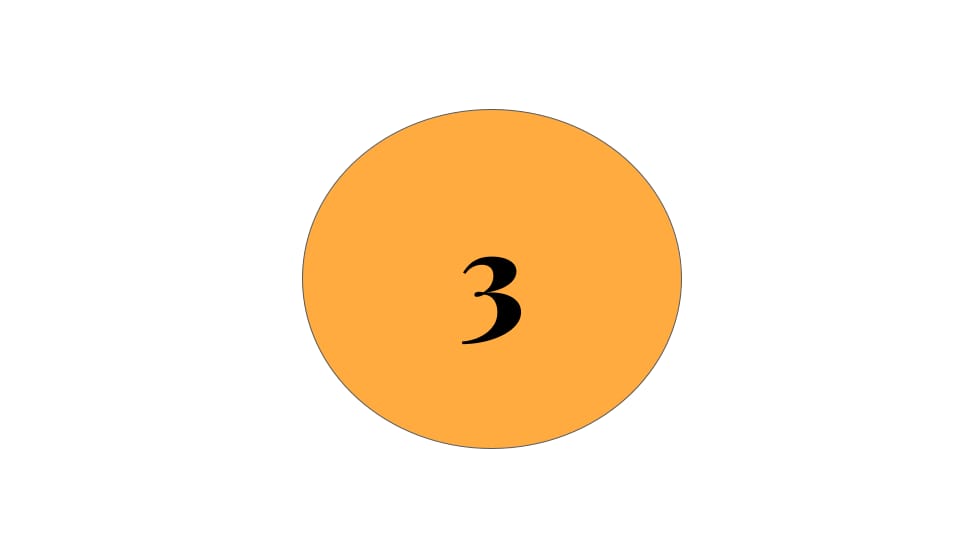
- May 13, 2024 : 4️⃣ Signals
May 13, 2024 : 4️⃣ Signals
Deep Dive into Postmarketing Report Checklists
4️⃣ Signals in the Nicotine and Tobacco Space
FDA and DOJ seized $700K of unauthorized e-cigarette product
Nicotine and tobacco-related FOIA analysis for April 2024
First TPSAC meeting announced for 2024 - June 26, 2024
FDA filed for scientific review modified risk tobacco product (MRTP) renewal applications submitted by Philip Morris Products S.A. for several IQOS products. - May 9, 2024

On April 30, the U.S. Food and Drug Administration (FDA), in coordination with the U.S. Department of Justice, announced that the U.S. Marshals Service seized unauthorized e-cigarette products valued at more than $700,000. The e-cigarettes were located in a warehouse in Alhambra, CA, and are believed to be owned by several California-based distributors. The seized products were mostly flavored, disposable e-cigarette products, including youth-appealing brands such as Puff Bar/Puff, Elf Bar/EB Design, Esco Bar, Kuz, Smok, and Pixi.
In a related development, Senator Richard Durbin (D. IL) extended a formal invitation for Brian Boynton, Principal Deputy Assistant Attorney General Civil Division and Brian King, Director CTP, to appear and testify on June 12, 2024 at a Senate Committee on the Judiciary hearing on the topic of the sale of unauthorized e-cigarettes.

20 nicotine and tobacco-related FOIA requests were closed in April 2024. Many of these requests were closed with no records found or denied outright. One request that was closed and presumably filled was for
“copies of all marketing denial orders (“MDOs”) the Food and Drug Administration (“FDA”) has issued for electronic nicotine delivery system (“ENDS”) products, and all material supporting such MDOs. This FOIA request creates a continuing obligation on FDA to supplement its response as additional MDOs for ENDS products are issued.”
A Tobacco Product Scientific Advisory Committee (TPSAC) hearing will be held for the MRTPA renewal of Swedish Match’s General Snus products modified risk granted orders issued under section 911(g)(1) - Risk Modification order on June 26, 2024 at FDA White Oak Campus, 10903 New Hampshire Ave., Bldg. 31 Conference Center, the Great Room (Rm. 1503), Silver Spring, MD 20993. The MRTP Application renewals were submitted to FDA CTP on July 17, 2023 You can read part of the redacted renewal request here.

On May 9, 2024, FDA filed for scientific review modified risk tobacco product (MRTP) renewal applications submitted by Philip Morris Products S.A. (PMP S.A.) for several IQOS products. The MRTP renewal was submitted on July 5, 2023. PMP S.A. is requesting a renewal of the MRGO - Exposure Modification for the IQOS products issued under section 911 (g) (2) of the FD&C Act. The MRTP Application renewals were submitted to FDA CTP on July 5, 2023 can read part of the redacted renewal request here.