- Smoke Signals: Navigating the Evolving Landscape of Nicotine and Tobacco Regulations
- Posts
- October 14, 2024 - Nicotine Pouch Literature Surveillance
October 14, 2024 - Nicotine Pouch Literature Surveillance
Issue #20 - Deep Dive: Data Standardization of Tobacco Product Submissions Using TIG Version 1.0

1️⃣ Nicotine and Tobacco-related Freedom of Information Act (FOIA) requests for September 2024 analyzed
2️⃣ Several new briefs were submitted to the docket of Supreme Court case No. 23-1038 in support of the respondent, Wages and White Lion Investments, L.L.C., dba Triton Distribution, et al.
3️⃣ Recent literature surveillance for oral nicotine pouch products
🤿 Deep Dive - Tobacco Implementation Guide Version 1.0 - Using Data Standards to Streamline Applications

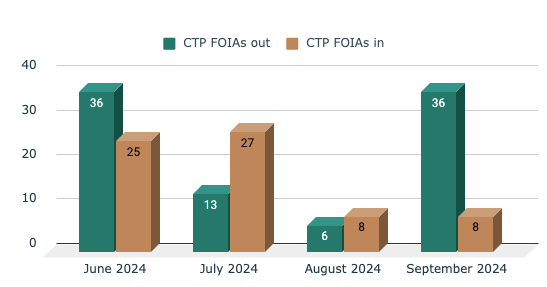
CTP FOIA Analysis for September 2024
Closed Denied / Partially Denied:
Requests for MDO letters and TPL reviews are often partially or fully denied.
Requests for specific, recent decisions (e.g., for SMOK, blu products) faced denials.
Highly specific requests for internal documents or recent decisions seem more likely to be denied.
Successful "Closed" Requests:
Requests for older or more general information tend to be successful.
Requests for contract information or public-facing documents appear more likely to be closed successfully.
Requests for scientific memos or guidance documents that are not tied to specific recent decisions seem to have a higher success rate.
Key Takeaway: There's significant month-to-month variation in both FOIA requests received ("CTP FOIAs in") and processed ("CTP FOIAs out").The number of FOIAs processed generally exceeds or matches the number received possibly due to a backlog.


Briefs in support of Wages and White Lion Investments, L.L.C., dba Triton Distribution (Respondents), et al. submitted. (No. 23-1038)
The Supreme Court brief filed by Wages and White Lion Investments on October 7, 2024, LLC presents a case between e-liquid manufacturers (Triton Distribution) and the FDA regarding the marketing authorization process for flavored e-cigarette products. The respondents argue that the FDA substantially changed its requirements for authorization without providing fair notice, failed to consider manufacturers' reliance on previous guidance, adopted new rules without proper procedures, and erroneously ignored marketing plans designed to prevent youth access.
A brief amici curiae was filed on October 8, 2024 in support of the respondents, Wages and White Lion Investments, by Vaping Industry Stakeholders.
An amicus brief was filed on October 10, 2024 in support of the respondents by the Washington Legal Fund.
Key Takeaway: These three briefs attempt to demonstrate a pattern of regulatory inconsistency and unfair treatment by the FDA, arguing that the agency's actions in denying marketing orders for the Respondents' flavored e-liquids were arbitrary and capricious. They urge the court to overturn the FDA's decision, citing due process concerns, lack of fair notice, and a disregard for the reliance interests of the Respondents. The Triton Appeal can be further interrogated using my Triton LLM Assistant here. I also created a short AI-generated podcast that discusses the history of tobacco regulation in the U.S. leading up to this court case.