- Smoke Signals: Navigating the Evolving Landscape of Nicotine and Tobacco Regulations
- Posts
- PMTA Registries and 2023 Annual Reports - February 20, 2024
PMTA Registries and 2023 Annual Reports - February 20, 2024
Issue # 03
An emergent issue for 2024 in the nicotine space has been the introduction of PMTA registry bills in state legislatures across the U.S. These proposed laws (listed below) will create directories of ENDS/vapor products on a state-level that have been certified by ENDS manufacturers. Each state bill is slightly different in wording but the intent is to require all manufacturers of alternative nicotine products or vapor products to pay a fee and certify with some frequency that they have (among other possible stipulations) submitted PMTAs for their nicotine vapor products on or before September 9, 2020. These proposed laws generally provide the state with additional enforcement authorities and prohibit the sale of any nicotine vapor product not listed in the directory.
Assuming some of these laws pass, which has already occurred in Louisiana and Oklahoma (vape attestation and registry lists here), the maintenance of a directory of ENDS products could prove to be a challenge over time. FDA CTP is the only stakeholder with the “master list” of deemed new tobacco products with timely applications to include/exclude from these directories. The published list of PMTAs submitted by September 9, 2020 includes over 15M individual SKUs. This list has not been updated since 2021. Even if assuming over 99% of these products have been rejected, there are still the 1M non-tobacco nicotine PMTAs submitted by May 14, 2022 for which a list has never been provided by CTP. These products would presumably not be certified in many instances and it is not clear how the products in the directories would be verified other than by manufacturer attestation. Also, there are several manufacturers in various stages of litigation which further confuses the lists for retailers. Creation and maintenance of such directories will be a challenge, requiring resources to be diverted from other potentially more effective enforcement efforts.
Altria, Philip Morris International and British American Tobacco released 2023 annual earnings reports in recent weeks. The transition to non-combustible products is obvious and there was a marked increase in references to “nicotine” as opposed to “tobacco” in each report. Nothing groundbreaking here except the speed at which it is occurring has accelerated in the last year. Forward-looking statements seemed to confirm that transition. I used Microsoft Copilot to provide a quick synopsis of each company’s report. This is another excellent use-case for the utilization of AI.
 |
 |
 |
PMTA
 | The U.S. Court of Appeals for the 5th Circuit granted R.J. Reynolds Vapor Co. a permanent stay of enforcement on the VUSE Alto, Vibe and Solo Menthol Pods which were issued market denial orders by CTP on October 12, 2023; January 24, 2023 and March 17, 2023 respectively. (February 5, 2024) British American Tobacco submitted an application to market its Glo Hyper Pro device as a reduced-risk product in the U.S. (February 8, 2024) BAT submitted a MRTP Application to FDA CTP for its heated tobacco device. The application could make claims on a reduced exposure or reduced risk claim. |
Select PMTA Registry bills introduced in 2024
Policy
 | Wave 8 data collection is currently underway and this new contract supports at least four additional waves of main data collection, as well as other special data collections. The new FDA Information Technology Strategy’s “Adopting Artificial Intelligence (AI) and Innovations” goal set a clear direction for the agency's approach to AI. As an important next step, ODT introduced the FDA AI Governance and Advisory Board to guide the agency through the complexities of AI integration. |
FDA released a draft for public comment titled The Scientific Integrity Policy of the U.S. Department of Health and Human Services. There are 7 specific areas of focus:
Protecting Scientific Processes
Ensuring the Free Flow of Scientific Information
Supporting Policymaking Processes
Ensuring Accountability
Protecting Scientists
Professional Development for Government Scientists, and
Federal Advisory Committees
Opinion
 | Under Dr. Gottlieb’s tenure a harm reduction platform for nicotine was promoted at FDA. In this clip he asserts that there is still a place for such conversation. With regard to Zyn nicotine pouch products, he states that the social media influencing is organic and not paid for by Philip Morris International, the manufacturer of Zyn. |
States across the country are commencing their legislative sessions, and public health advocates have seen a resulting wave of so-called “registry” bills that have been predominantly supported by the tobacco industry. These laws seek to establish or amend product registries or directories that purport to regulate retail sales of e-cigarette and other nicotine products. Generally speaking, these laws limit the products being marketed to only those that the FDA has approved for sale—or, more accurately, to only those products that the FDA has not definitively prohibited for sale.
Recent Publications of Interest
 | Challenges in legitimizing further measures against smoking in jurisdictions with robust infrastructure for tobacco control: how far can the authorities allow themselves to go? (January 28, 2024) Karl Erik Lund and Gunnar Saebo If people who smoke are aware of and have accepted the risks, are willing to pay the price, smoke exclusively in designated areas, and make decisions uninfuenced by persuasive messages from manufacturers – a further tightening of anti-smoking measures may, from a legiti-mation perspective, appear challenging for regulators. Lund and Saebo |
This cross-sectional study found a substantial increase in youth e-cigarette use prevalence in the early 2010s, peaking in 2019 and declining during the early 2020s to still-concerning levels. From 2021 to 2022, use increased for certain groups, such as Hispanic and non-Hispanic Black youths. Temporal e-cigarette use trends by age, sex, and race and ethnicity highlight disparate groups that require tobacco prevention services. Our study limitations include potential concerns about recall bias and the inability to examine trends between 2020 and 2021.
ENDS PMTA Marketing Denial Order Litigation Appeals Tracker (non-exhaustive)
This is an ever-evolving list of appeals in the various U.S. Circuit Court of Appeals, with the most recent (as of this publishing) being the Bidi Vapor appeal in the 11th Circuit Court of Appeals on January 26, 2024.
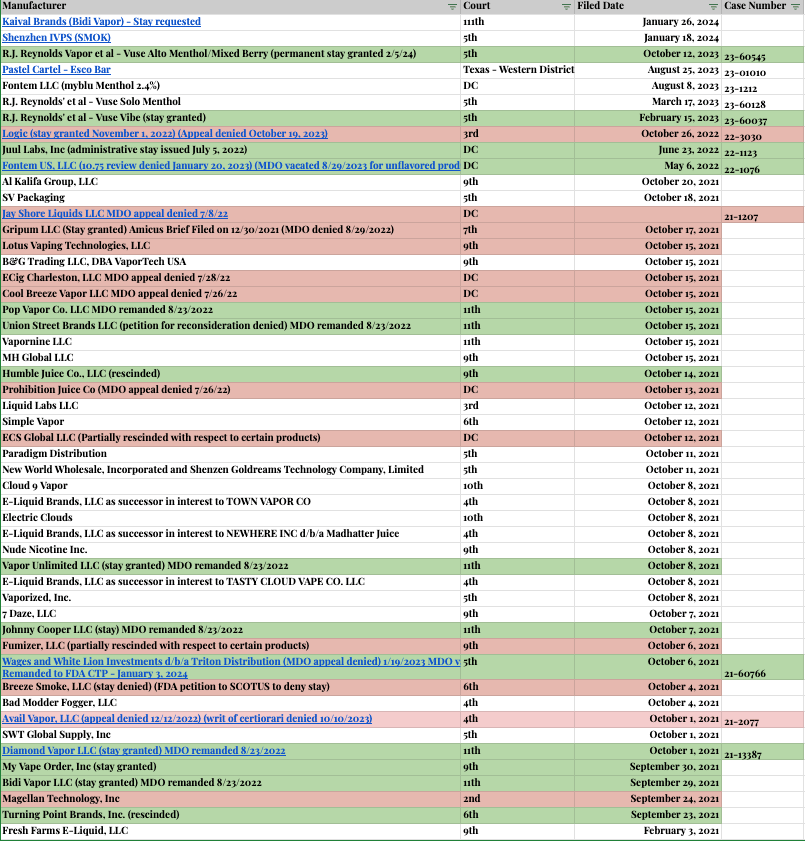
ENDS PMTA MDO Appeals as of February 2024
Resources
J.E. Dice Regulatory Solutions weekly newsletters - These are AI-generated weekly data dumps, probably 100 articles per week in trade press and literature publications. |
