- Smoke Signals: Navigating the Evolving Landscape of Nicotine and Tobacco Regulations
- Posts
- September 16, 2024 - National Youth Tobacco Survey (NYTS) data released
September 16, 2024 - National Youth Tobacco Survey (NYTS) data released
Issue #18 - Deep Dive - FDA v Triton SCOTUS case
Over the last several months I’ve written articles on LinkedIn about various aspects of the regulatory strategy and intelligence process in the nicotine and tobacco space. These articles are high-level overviews that introduce concepts I feel are important foundations to building a robust regulatory intelligence program. I’ve collected them at my website as well for easier reference.
Results from the National Youth Tobacco Survey (NYTS) for 2024 released
Brian King, Director of Center for Tobacco Products (CTP), testified before the Energy and Commerce Committee and provided an update to PMTA review activities.
Enforcement policy update for required warnings for cigarette packages and advertising
Deep Dive - Knowledge graph for FDA v Triton U.S. Supreme Court Case

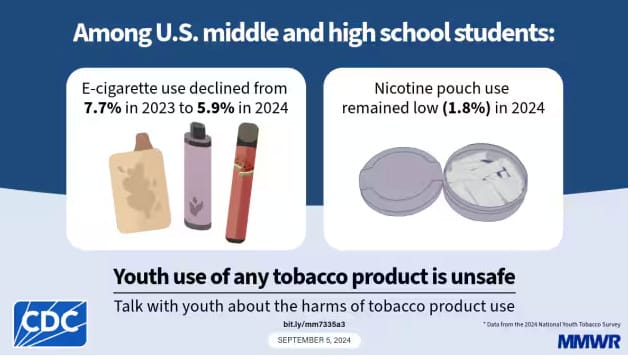
During 2023–2024, current e-cigarette use among middle and high school students declined from 7.7% to 5.9%. Current nicotine pouch use (1.8%) did not change significantly during this period.
Future NYTS milestones:
National Youth Tobacco Use survey (NYTS) Milestone | Estimated Start Date | Estimated Completion Date |
|---|---|---|
Public use data set release (2024 data) | 6/1/24 | 11/30/24 |
Develop the 2025 survey instrument | 10/1/24 | 12/31/24 |
Start fielding 2025 survey | 1/15/25 | 5/31/25 |
Public use data set release (2025 data) | 6/1/25 | 11/30/25 |
Key Takeaway: 44% (720,000) of students currently using e-cigarettes rate their frequency of use at 1-5 days per month. 53.7% (250,000) of students currently using nicotine pouches rate their frequency of use at 1-5 days per month. These cohorts, respectively, are the largest for each product category and indicate that most students who use these nicotine products are not daily users.


On September 10, 2024 the Committee on Energy and Commerce held a subcommittee hearing where Brian King, Director, Center for Tobacco Products, testified about the progress CTP has made over the past 15 years. Several highlights from King’s testimony:
Voiced support for H.R.9425, a recently introduced bill which seeks to amend the Federal Food, Drug, and Cosmetic Act to authorize tobacco user fee assessments for all regulated tobacco products (including e-cigarettes), and for other purposes.
Stated around 500,000 ENDS PMTAs remain in queue for CTP to review.
Committed to eventually getting to a 180-day turnaround for PMTA review cycles.
CTP is 100% funded by user fees, which have not been updated by Congress to reflect the realities of the current tobacco product marketplace. The agency's fiscal year 2025 budget, includes an additional 114 million dollars in tobacco user fees indexed for inflation and a request for authority to assess user fees for manufacturers and importers of all regulated tobacco products including e-cigarettes. These additional resources will help us achieve more including in the areas of enforcement and new product review, which I know where areas of priority for members of this committee.
…for the 114 million, we proposed educating about half to enforce them in compliance, 25% application review and about the remaining 25 for education to the public, which could include produced risk alternatives to smoking.
Key Takeaway: Several lawmakers challenged King on the issue of CTP requesting additional user fee dollars when the Center is 100% funded by industry with no Congressional spending oversight. “Until they know better how dollars are prioritized and have agreement on those priorities, it is premature to provide any more funding” - Brett Guthrie (R-KY). There was also discussion on creating a more transparent and predictable regulatory process which presumably involves getting to a 180-day application review cycle. Certain PMTAs have been in CTP review queues for several years. This was similarly the case for SE Reports in the mid 2010s.