- Smoke Signals: Navigating the Evolving Landscape of Nicotine and Tobacco Regulations
- Posts
- September 3, 2024 - CTP User Fees Update
September 3, 2024 - CTP User Fees Update
Issue #17 - (Deep Dive) Prompt Engineering for LLM Success

FDA CTP user fee collection updates through June 2024
FDA filed a brief with the Supreme Court of the United States in FDA vs Triton Distribution, et al. (23-1038)
An administrative law judge from the U.S. International Trade Commission (ITC) ruled in favor of JUUL Lab Inc’s complaint against NJOY and recommended an exclusion order that would prohibit the importation of NJOY ACE products into the U.S.
FDA issued a final rule that raises the minimum age for certain restrictions on tobacco products.
Deep Dive - Prompt Engineering for LLM Success 🤖

FDA Center for Tobacco Product User Fees
The FDA is authorized to collect user fees from manufacturers of six classes of tobacco products: cigars, pipe tobacco, cigarettes, snuff, chewing tobacco, and roll-your-own tobacco. E-cigarettes are currently not included in this list, which has been a point of discussion in recent budget proposals.
The FDA uses these metrics to monitor their progress towards meeting the statutorily required collection amount and ensures they are on track to fully fund their tobacco regulation activities.
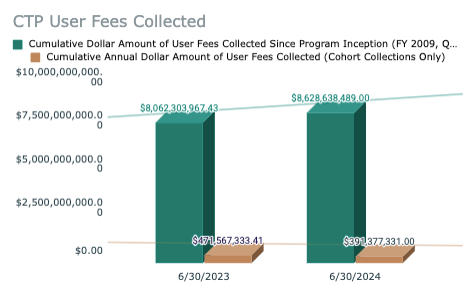
💵 FDA Center for Tobacco Products User Fees Update through June 30, 2024:
🟢 Cumulative Annual (YTD) Dollar Amount of User Fees Collected (Cohort Collections Only)
➢Fiscal YTD 2024 through June 30, 2024 - $391,277, 331
➢Fiscal YTD 2023 through June 30, 2023 - $471,567,333
Collections down ~$80M when compared to the same point in FY2023
🟢 Cumulative Dollar Amount of User Fees Collected Since Program Inception (FY 2009, Quarter 4)
➢$8,628,638,489
Takeaway: FDA CTP operations are 100% funded by industry. YTD 2024 collections are down ~18% signaling a drop in combustible cigarette volumes which make up the bulk of CTP-regulated company earnings. This would be in line with recent manufacturer quarterly earnings releases which have shown significant combustible cigarette volume declines.


Supreme Court of the United States receives FDA Petitioner response
The Fifth Circuit concluded that FDA had imposed a categorical ban on flavored e-cigarettes. But FDA’s denial orders make no reference to any such ban, showing instead that FDA considered each application on an individual basis. And while the petition for a writ of certiorari in this case was pending, FDA granted applications for authorization to market four flavored e-cigarette products—a step it could not have taken if it had subjected such products to a categorical ban.
Finally, the Fifth Circuit concluded that FDA had acted arbitrarily by authorizing other manufacturers’ menthol-flavored e-cigarette products, but refusing to authorize respondents’ flavored e-cigarette products. As respondents have conceded, that conclusion was wrong. At the time of the court’s decision, FDA had not actually authorized any menthol-flavored e-cigarette products. And although FDA has since authorized four such products, it did so only after evaluating them under the same framework that it has applied to other flavored e-cigarettes.
In summary:
Firstly, the FDA contends that the appeals court wrongly concluded that the FDA unlawfully departed from previously announced evidentiary standards when evaluating the applications for flavored e-cigarette products.
Additionally, the FDA asserts that the appeals court's requirement for the FDA to notify applicants in advance of how it planned to evaluate flavored e-cigarette products went beyond what is demanded by the Administrative Procedure Act (APA) and disregarded the agency's discretion to develop regulatory standards through case-by-case adjudication.
Furthermore, the FDA argues that the appeals court erred by concluding that the FDA had acted arbitrarily by authorizing other manufacturers' menthol-flavored e-cigarette products but refusing to authorize respondents' flavored e-cigarette products, which the FDA contends was incorrect.
The FDA maintains that its denial orders easily satisfy the deferential arbitrary-and-capricious standard and that the appeals court's identified flaws in the FDA's reasoning lack merit.
Takeaway: FDA argues that with the recent authorization of NJOY ACE and Daily Menthol-flavored ENDS products there is no categorical ban on flavored e-cigarettes as claimed.