- Smoke Signals: Navigating the Evolving Landscape of Nicotine and Tobacco Regulations
- Posts
- Tobacco Retailer Compliance Checks and MDO updates - March 18, 2024
Tobacco Retailer Compliance Checks and MDO updates - March 18, 2024
Issue #5
People with high expectations have very low resilience.
Much of what currently drives nicotine and tobacco regulatory policy in the U.S. centers around underage tobacco-use prevention. Device age-gating technology has also become a recent focal point of premarket application development. The other pillar of underage-use prevention is through compliance activities. CTP’s Office of Compliance and Enforcement (OCE) coordinated over 100K brick and mortar tobacco retailer for underage purchaser (UP) inspections in FY23 and recently released that data. Source data is located here.
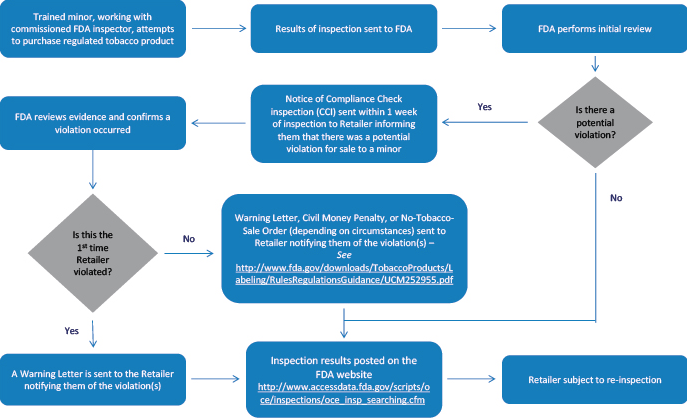
Tobacco retailer UP process
The FY23 data was then analyzed across Brand and by State.
The state-level data shows an underage purchase success-rate of ~16% across the U.S. Some states of note were Virginia at 32.67% and Utah at 2.45%.
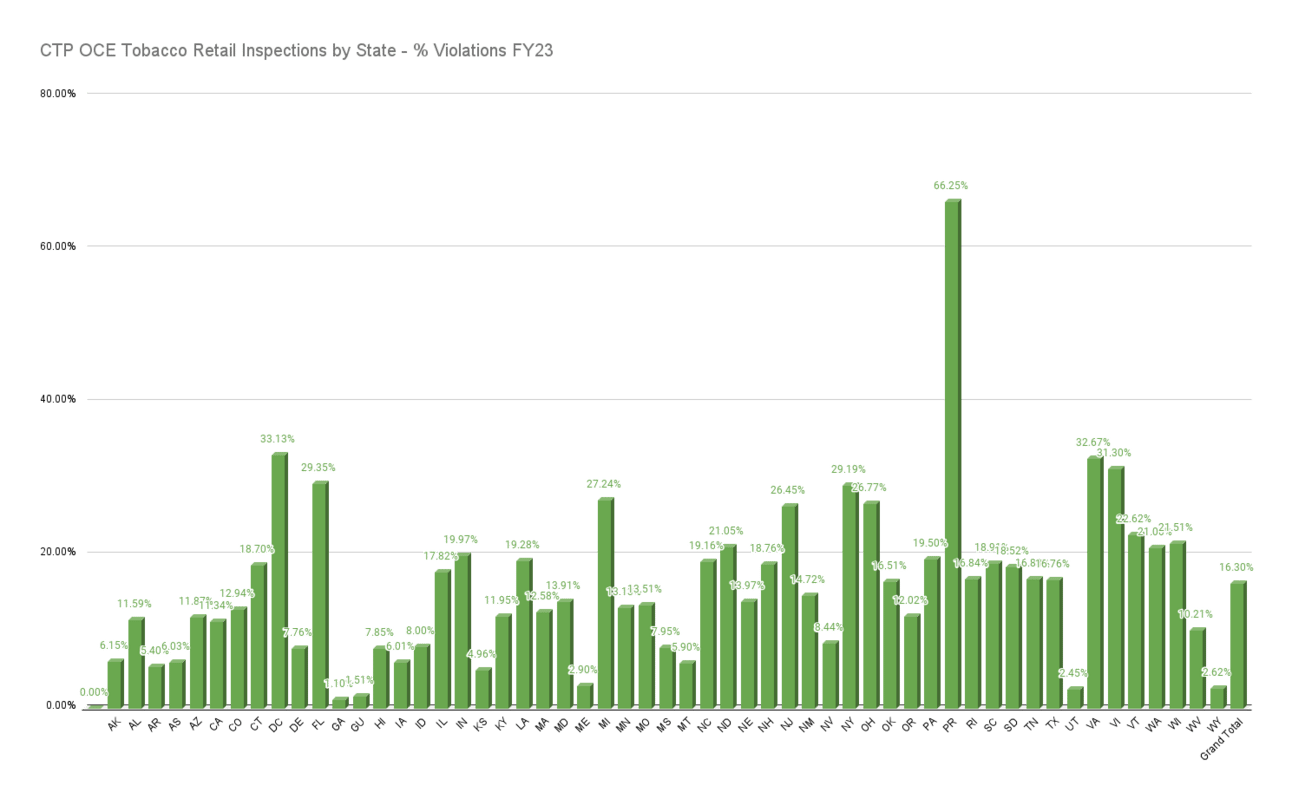
UP success rate by State
When evaluating the compliance data by brand, Swisher at 19.9% and Black & Mild at 13.6% have the highest UP success rate for tobacco products. The major limiting factors of this data are the large number of uncategorized ‘Other’ brands as well as a lack of data for total attempts by brand. For example, there were 875 successful UP attempts for JUUL-brand but the data does not indicate the total number of attempts for the JUUL-brand products.
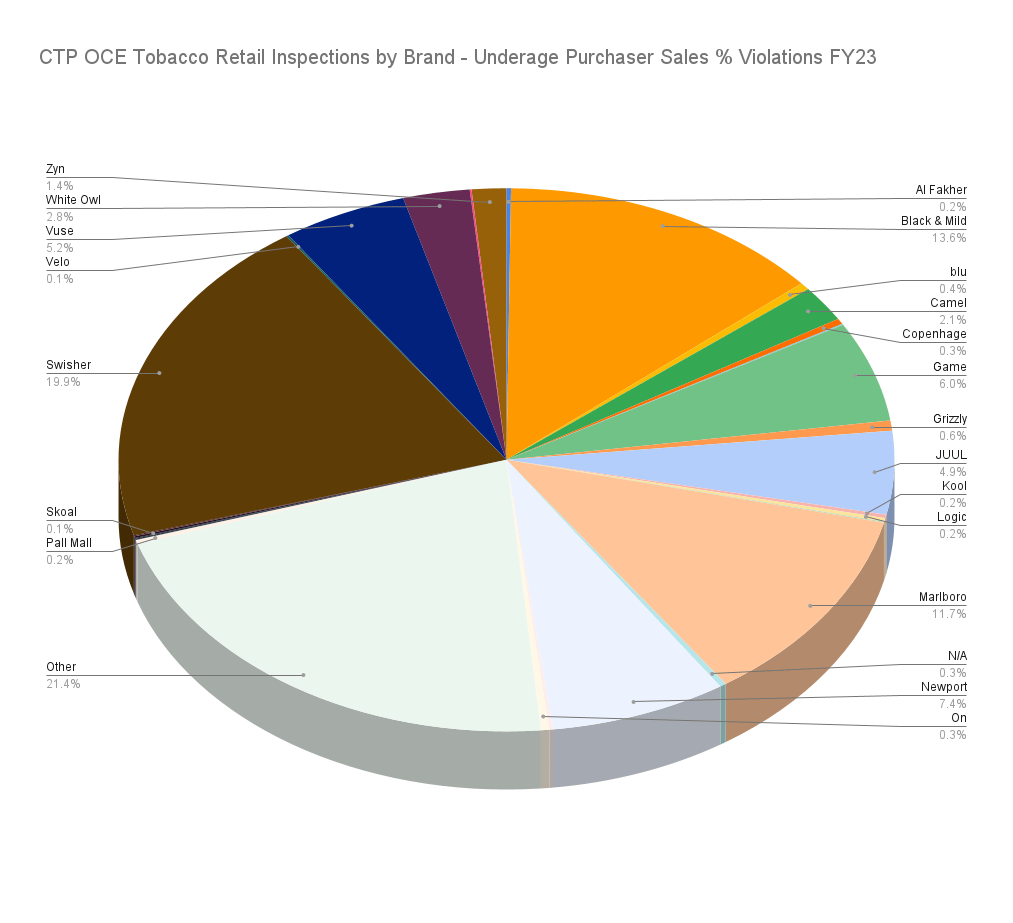
UP success by brand. Percentage of total
It is important to recognize the tools that are currently available to prevent underage tobacco-purchase and consider bolstering efforts to improve the processes already in place. While the PMTA registry bills winding their way through various U.S. state legislatures address a peripheral issue (noncompliant product) to UP, it is important to consider how these new laws create additional confusion and unnecessary disruptions to an already-regulated marketplace.
PMTA
 | On February 27, 2024 the U.S. Court of Appeals 10th Circuit denied Electric Clouds, Inc. and Cloud 9 Vapor Products, LLC MDO appeals. (February 27, 2024) These MDO appeals (21-9577 and 21-9578) were originally filed in October 2021. |
The FDA didn’t mislead Electric Clouds or Cloud 9 about what to put in their applications, and the FDA could reasonably regard the literature reviews and customer surveys as inadequate. Along with the literature reviews and customer surveys, Electric Clouds and Cloud 9 submitted marketing plans. Though the FDA didn’t review these marketing plans, any possible error would have been harmless. These plans relied on youth-prevention measures that the FDA had previously rejected as ineffective. We thus deny the petitions for judicial review of the denial of the applications to market flavored e-liquids.
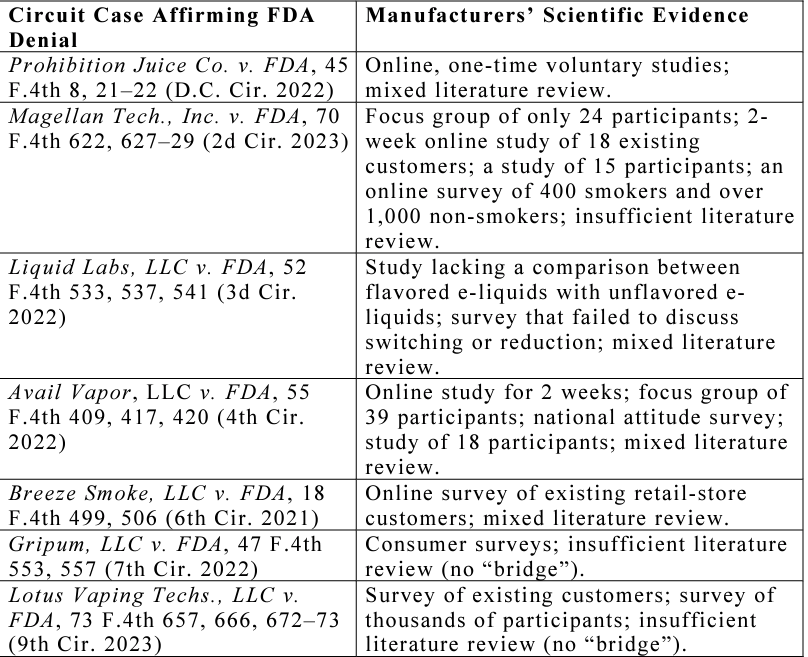
Included in the Cloud 9 decision were several previous confirming denials from other Courts
Additional Marketing Denial Orders (MDOs) were issued by CTP in February 2024. 3 of the 5 companies had MDOs issued in 2021 as well. It is possible that these were resubmitted PMTAs with a synthetic nicotine source after a refuse to accept decision in 2021.
Manufacturer | Date of MDO Issued |
|---|---|
LDP Designs LLC | 2/20/2024 |
VR Labs LC | 2/8/2024 |
Stark Vapor LLC | 2/8/2024 |
eCig Source | 2/8/2024 |
BLB LLB dba The Vape Mall | 2/8/2024 |
The U.S. House and Senate recently sent letters to stakeholders including FDA and several convenience store chains, identifying gaps in TCA policy and enforcement. The House letter focused on FDA’s unwillingness/inability to authorize more smoke-free products while the Senate letters focused on the compliance and enforcement issues for tobacco products at retail.
Every single e-cigarette or vaping product that is not on this list was not authorized by FDA, making such products illegal and adulterated and misbranded under Sections 902 and 903 of the Federal Food, Drug, and Cosmetic Act. Companies distributing or selling any such unauthorized products are subject to enforcement penalties by the FDA, which may include a civil monetary penalty or no-tobacco-sale order. Richard Durbin |
U.S. Representative Richard Hudson (R-NC) led 60 of his House Republican colleagues in a letter to President Joe Biden demanding the Food and Drug Administration (FDA) expedite the approval process for smoke-free tobacco products so American consumers can have more choices in alternative products. |
Policy and Enforcement

Dr. Brian King, Director of CTP, January and February 2024 met with stakeholders from several groups in January and February 2024. FDA officials regularly meet with stakeholders from industry, academia and government. Tobacco manufacturers, importers, researchers, and/or investigators who seek meetings with the Office of Science within the Center for Tobacco Products regarding their research and development plans related to tobacco products can consult guidance here.
Event Date: 02/05/2024
Location: Panama City, Panama
Subject: Conference of Parties to the WHO Framework Convention on Tobacco Control
FDA Participant/Group: Several FDA/HHS staff
Non FDA Participant/Group: Several representatives of the Conference of Parties to the WHO Framework Convention on Tobacco Control
Event Date: 01/29/2024
Location: Silver Spring, MD
Subject: Listening Session; Juul Labs Inc. (JLI)
FDA Participant/Group: Several FDA staff
Non FDA Participant/Group: Several representatives of JUUL Labs, Inc.
Event Date: 01/12/2024
Location: Virtual
Subject: Tobacco Enforcement FDA Participant/Group: Several FDA staff Non FDA Participant/Group: Several staff from the Senate Committee on Health, Education, Labor and Pensions (HELP)
Event Date: 1/10/2024
Location: Virtual
Subject: Premarket Tobacco Product Application (PMTA) Updates and Enforcement Issues
FDA Participant/Group: Several FDA staff Non FDA Participant/Group: Several staff from House Committee on Oversight and Accountability (HOA)
Opinion
 | While most agencies rely on at least a portion of their appropriations from Congress to operate, providing increased transparency and accountability into expenditures, the FDA’s Center for Tobacco Products (CTP) is unique in being entirely user-fee-funded. The Center’s statutory mandate is to regulate the tobacco industry, whose products are expressly authorized under law, in an efficient, lawful, and objective manner. But is this really how those user fees are being spent? The agency isn’t sending tobacco user fees to special interest organizations whose raison d’etre is to put the entire tobacco industry out of business, is it? Michael Chamberlain |
Recent Publications of Interest
Karey E, Xu S, He P, Niaura RS, Cleland CM, Stevens ER, Sherman SE, El-Shahawy O, Cantrell J, Jiang N. Longitudinal association between e-cigarette use and respiratory symptoms among US adults: Findings from the Population Assessment of Tobacco and Health Study Waves 4-5. PLoS One. 2024 Feb 29;19(2):e0299834. doi: 10.1371/journal.pone.0299834. PMID: 38421978; PMCID: PMC10903800.
Partial Abstract
Background: We assessed longitudinal effects of e-cigarette use on respiratory symptoms in a nationally representative sample of US adults by combustible tobacco smoking status.
Conclusions: The association between e-cigarette use and respiratory symptoms varied by combustible tobacco smoking status. Current combustible tobacco smokers who use e-cigarettes have an elevated risk of respiratory impairments.Karey E, Xu S, He P, Niaura RS, Cleland CM, Stevens ER, Sherman SE, El-Shahawy O, Cantrell J, Jiang N. Longitudinal association between e-cigarette use and respiratory symptoms among US adults: Findings from the Population Assessment of Tobacco and Health Study Waves 4-5. PLoS One. 2024 Feb 29;19(2):e0299834. doi: 10.1371/journal.pone.0299834. PMID: 38421978; PMCID: PMC10903800.
5 corrections were made by the author to the following article.
Alzahrani T (November 06, 2023) Electronic Cigarette Use and Myocardial Infarction. Cureus 15(11): e48402. doi:10.7759/cureus.48402Abstract
This article has been corrected by the Editor-in-Chief. The online and PDF versions have been updated accordingly. The corrections are as follows:
The following sentence in the Abstract has been corrected as follows: "This study suggests that current e-cigarette use increases the risks of cardiovascular disease, including myocardial infarction and stroke, in subjects who never smoked cigarettes." corrected to "This study suggests that current e-cigarette use may increase the risks of myocardial infarction in subjects who never smoked cigarettes."
The following sentence in the Discussion section has been corrected as follows: "The results of this study suggest that current e-cigarette use is associated with myocardial infarction." corrected to "The results of this study suggest that current e-cigarette use may be associated with myocardial infarction."
The following sentence in the Conclusions section has been corrected as follows: "E-cigarettes are associated with myocardial infarction in subjects who have never smoked cigarettes." corrected to "E-cigarettes may be associated with myocardial infarction in subjects who have never smoked cigarettes."
Reference 18 has been updated to note that the referenced article has been retracted: Bhatta DN, Glantz SA: Electronic cigarette use and myocardial infarction among adults in the US population assessment of tobacco and health. J Am Heart Assoc. 2019, 8:e012317. 10.1161/JAHA.119.012317
The paragraph following Table 2 has been corrected so that the odds ratios match those listed in Table 2.
An interview was posted with Dr. Arielle Selya on youth e-cigarette use. Interesting discussion of gateway theory of e-cigarette to combustible cigarette use @10:00.
Clinical Trials
Reference cigarettes have been available for researchers in the nicotine and tobacco space for many years (3R4F, 1R6F). Standardized Research Electronic Cigarette (SREC) devices are designed to produce aerosols that mimic the characteristics of those produced by other e-cigarette products, making them useful for comparative studies of e-cigarette aerosol exposure.
 | Pharmacokinetics and Pharmacodynamics of Nicotine With Use of Standardized Research Electronic Cigarette (SREC) (SREC22) (NCT05658471) This is a crossover study that will examine use behaviors, chemical exposures, and biological effects of Standardized Research Electronic Cigarette (SREC) compared to usual brand e-cigarette use in natural or synthetic nicotine users. |
Arms | Sponsors and Collaborators | Estimated Study Completion Date |
|---|---|---|
Usual Brand e-cigarette then SREC SREC then Usual Brand e-cigarette | University of California, San Francisco National Institute on Drug Abuse | January 31, 2025 |
Resource Links
CTP Tobacco Problems Dashboard (new 3/18) |

