- Smoke Signals: Navigating the Evolving Landscape of Nicotine and Tobacco Regulations
- Posts
- TPE 2024 and recent news - February 5, 2024
TPE 2024 and recent news - February 5, 2024
Total Product Expo 2024 and UBS Global Tobacco Report among other industry news
Issue # 02

I had the opportunity to attend the Total Product Expo 2024 in Las Vegas last week. Chemular hosted an educational event at TPE titled Fortify Your Future. This event brought together many manufacturing, scientific, regulatory and legal experts in the field of nicotine, tobacco and harm reduction. The event provided a comprehensive overview of the current state of the industry as well as strategies to minimize the damage being inflicted by illicit product distribution and incoherent regulatory decisions. Thoughtful consideration was provided by knowledgeable stakeholders to where the industry might be headed in the next several years. I saw many former colleagues and friends for the first time after probably the most devastating 18 months for business closures and job losses in the industry. The future does, in fact, belong to those who show up and it was refreshing to see so much enthusiasm even after so much setback. Here is a rundown of my high-level takeaways from several of the presentations.
Industry Outlook:
Regulatory, business, and operating environment insights were provided by Chris Greer from TMA.
Discussion on flavors, menthol bans, and the impact of political changes on regulations (pendulums swing both ways).
Four Pillars of PMTA Strategy by Lillian Ortega:
Legal considerations, scientific foundations, manufacturing processes, and marketing strategies discussed.
Emphasis on abuse liability, brand reputation, and engaging in the PMTA process.
Compliance is slow but over 300 warning letters issued in 2023 which is higher than previous years.
Application Pitfalls by Stacy Ehrlich:
Detailed stages of FDA application failure, from refuse to file, refuse accept to deficiency letters.
Emphasis on the importance of switching studies in PMTAs now.
Essential Values of Good Suppliers by Bonnie Coffa and Mike Bond:
Criteria for lab selection and the consequences of wrong testing.
Changing suppliers considerations and transition planning to new suppliers is important.
Panel Discussion:
Pain points for clients include vague communication with FDA CTP, compliance for new regulations introduced after submissions and incentives being backwards. The cost/benefit ratio is out of order.
Tech stack at FDA CTP is improving to include more listening sessions and industry being considered as stakeholders rather than victims of the process.
Marketing and Product Intelligence by Tim Phillips:
Overview of disposable vape market trends.
Insights into intoxicating hemp-derived cannabanoids (IHDC) and THC caps in hemp products.
Making the Switch by Brad Seipel:
Overview of innovation in the nicotine market.
Survey-based research insights and considerations for different consumer demographics.
The last 10-15 years have been an explosion of innovation after years of stagnation in the industry.
Creating Great Products in an Increasingly Regulated Market by Henry Sicignano:
Success story of creating Spree Bar using Metatine, a non-nicotine alkaloid.
Emphasis on rechargeable and reusable technology.
The Growth of Nicotine Pouches and other Innovative Products by David O’Neill:
First-mover advantage is a thing in the nicotine pouch category.
Comparison of mature vs nascent markets in the US versus EU.
The impact of AI on product development of flavor combinations in the market.
FDA Challenges and Practical Implications by Bryan Burd:
Overview of the various stakeholders at play in the market, including FDA, lawmakers, industry, and illicit market participants.
Strategies for navigating PMTA, NTN, and new applications include submitting minimum viable product PMTAs with rolling amendments and keeping FDA CTP informed of ongoing studies and open lines of communication with RHPMs.
TPE 2024 Educational Sessions sponsored by Chemular
UBS released a report titled - Global Tobacco - Tobacco’s Transformation likely to slow. The key takeaway is that next-generation products (NGP) contribution revenue in industry, currently around 20% of total revenue per year, is likely to slow over the next 5 years. This report is an equities analysis of worldwide nicotine and tobacco industry trends. Very dense with information and highlights the evolution of NGPs over different geographies. I found this chart to be extremely helpful when keeping track of who’s manufacturing what in the NGP space.
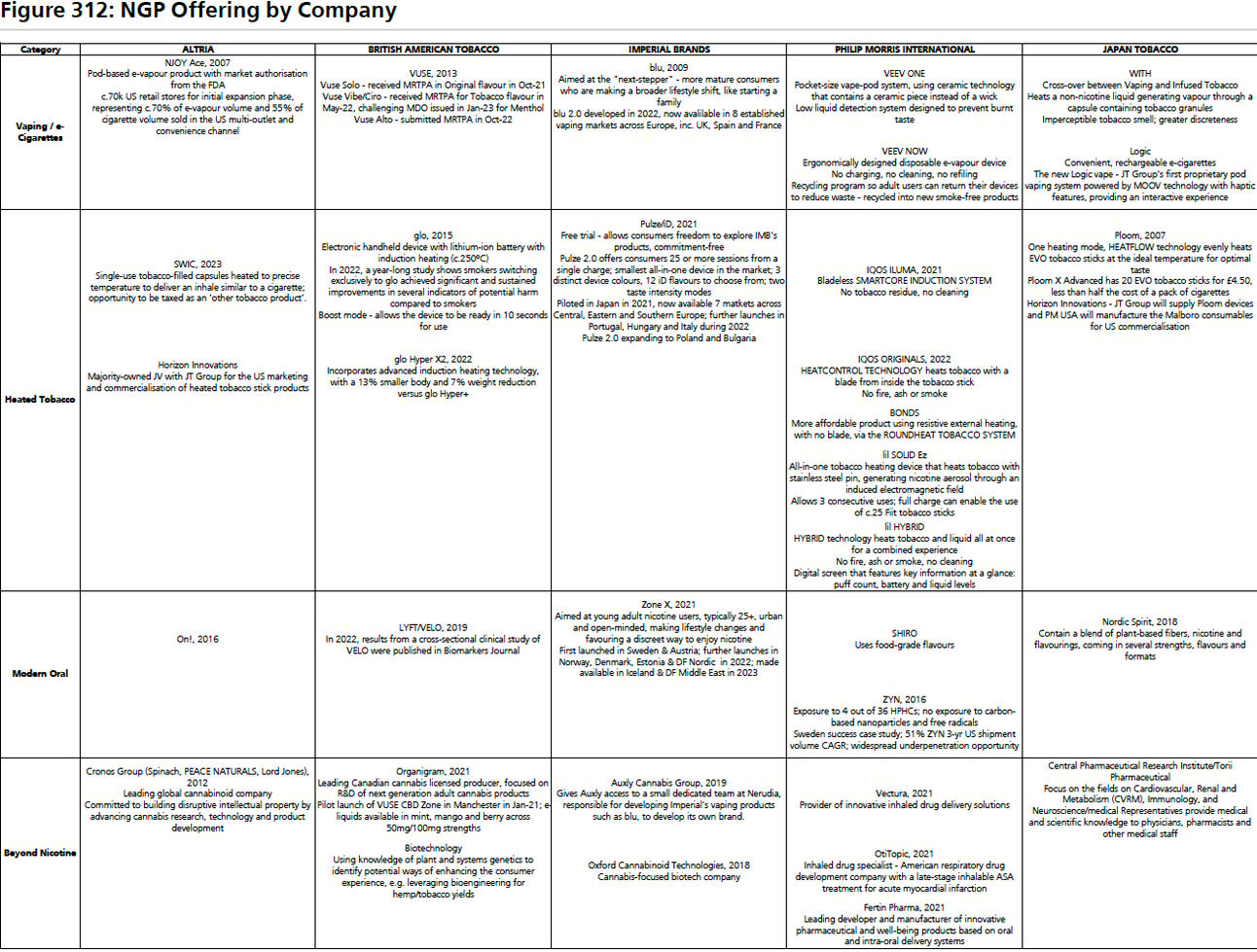
PMTA
 | FDA Denies Marketing of Bidi E-Cigarette (January 22, 2024) FDA CTP begins 2024 by announcing the marketing denial orders for several covered applications (Suorin and blu PLUS+ on January 19, 2024 and SMOK devices on January 16, 2024) in advance of the new June 30, 2024 deadline to have all September 2021 PMTAs reviewed. Takeaway: These MDOs indicate the CTP is not only denying “tobacco” flavored products but also, in the case of SMOK, denying ENDS devices without e-liquids associated. |
FDA Seeks $20K+ Fines Against Retailers Selling Unauthorized Youth-Appealing E-Cigarettes (January 30 2024)
FDA has issued complaints for civil money penalties (CMPs) against 21 brick and mortar retailers for the sale of unauthorized Esco Bars e-cigarettes, a popular youth-appealing brand.
Takeaway: Enforcement efforts continue to accelerate with 22 warning letters issued so far in 2024.
Policy
 | FDA released a guidance document titled - Conducting Remote Regulatory Assessments (RRA) Questions and Answers (February 2024) FDA is issuing the draft guidance to describe the Agency’s current thinking regarding its use of remote regulatory assessments (RRAs) in order to increase industry’s understanding of RRAs. No 483s will be issued during an RRA. |
Opinion
 | Chuck Schumer Attacks Lifesaving Zyn Nicotine Pouches (January 24, 2024) - Guy Bentley The most worrying aspect of Schumer's demonization of Zyn is that it contributes to the false impression that just because something contains nicotine, it's a threat to public health. What makes cigarettes so lethal is not nicotine but setting tobacco on fire and inhaling the smoke. Guy Bentley |
Altria: The Great Race (February 4, 2024) - Devin Lasarre
A common, and I believe incorrect, train of thought is that vapes are accessible, come in wider ranges of flavor, do not produce lingering smoke or smell, and categorically carry a lower risk profile, and therefore, there is no reason for anyone to not ditch cigarettes. But for all of their virtuous qualities, vapes simply do not perfectly replicate the experience of smoking a cigarette.
Recent Publications of Interest
 | UCSF and Truth Tobacco Industry Documents just released its JUUL LABS COLLECTION. This collection contains documents related to the e-cigarette brand JUUL and come from a number of sources. This is a newly-released set of documents and contains deposition interviews, certain FOIA requests made to JUUL Labs Inc as well as market research and publication documents. |
ENDS PMTA Marketing Denial Order Litigation Appeals Tracker (non-exhaustive)
This is an ever-evolving list of appeals in the various U.S. Circuit Court of Appeals, with the most recent (as of this publishing) being the Shenzhen IVPS Technology Co., Ltd., (SMOK brand) appeal in the 5th Circuit Court of Appeals on January 19, 2024.
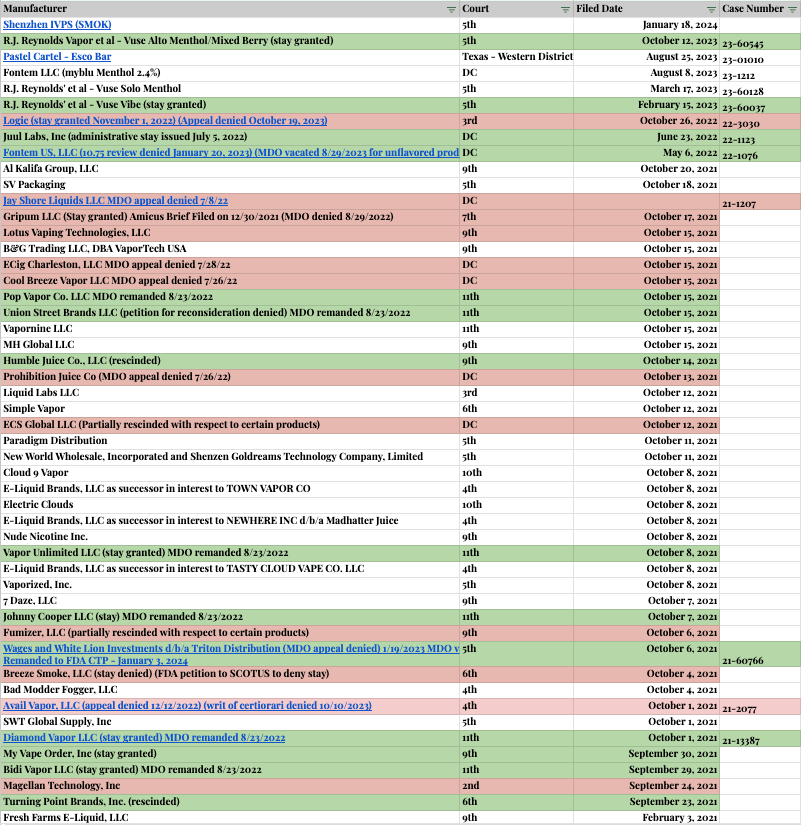
ENDS PMTA Marketing Denial Order Appeals
Select Dates for Acceptance/Filing Review for Various PMTAs as of January 23, 2024
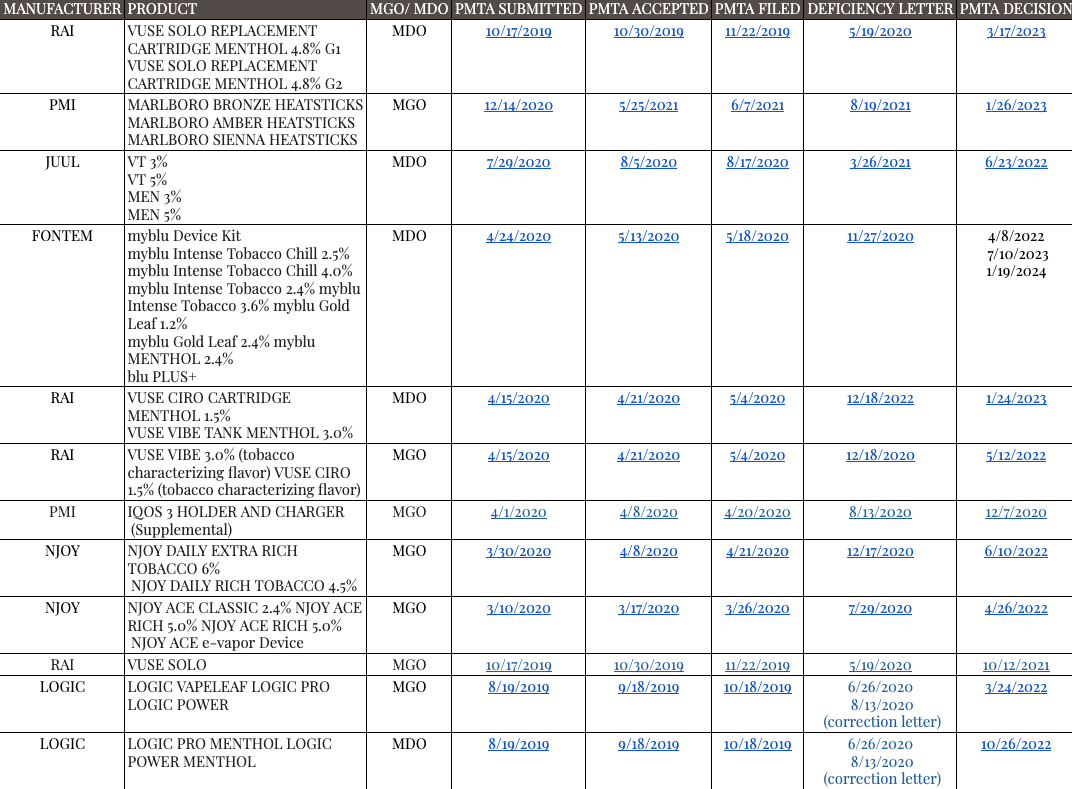
Select Dates for Acceptance/Filing Review for Various Covered PMTAs as of January 23, 2024
Covered PMTA Timelines
Average time from submission to decision is 2.5 years.
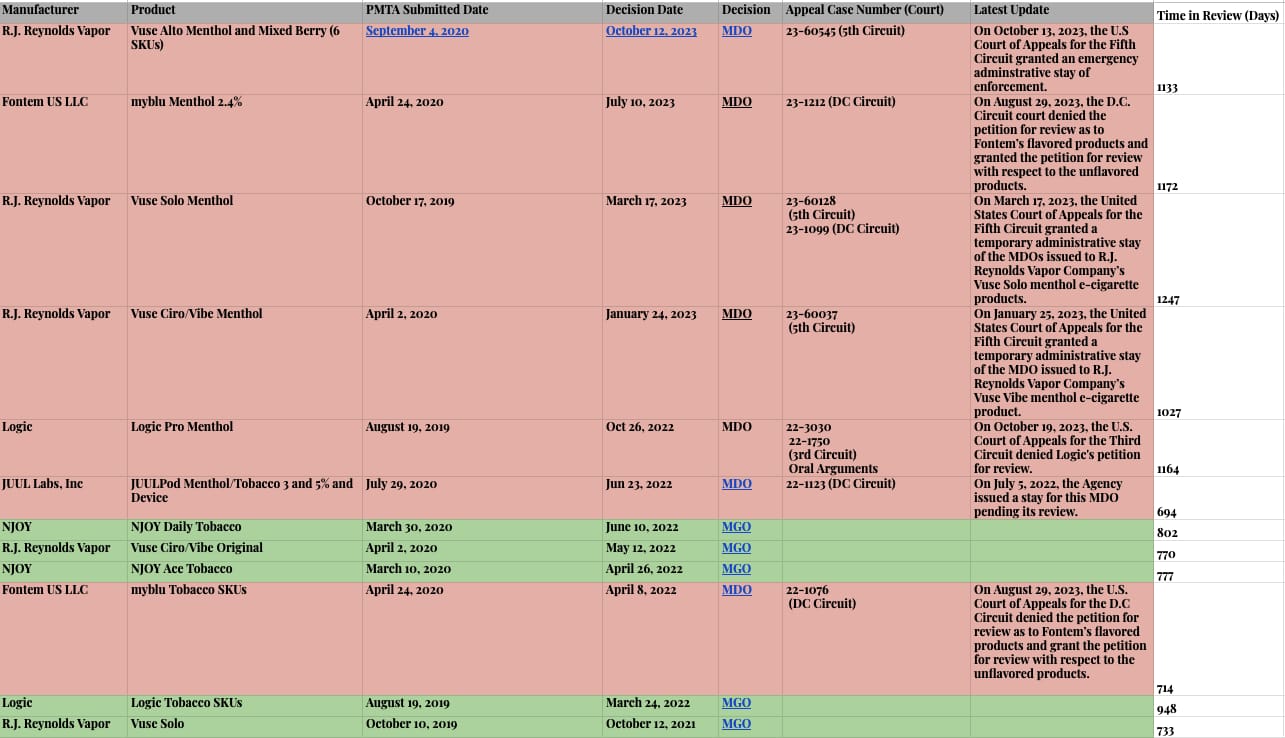
Covered PMTA Timelines as of January 2024
Resource Links
J.E. Dice Regulatory Solutions weekly newsletters - These are AI-generated weekly data dumps, probably 100 articles per week in trade press and lit publications. |